当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Inverted Region in Electrochemical Reduction of CO2 Induced by Potential-Dependent Pauli Repulsion
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2023-06-09 , DOI: 10.1021/jacs.3c02447 Leyu Liu 1 , Hai Xiao 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2023-06-09 , DOI: 10.1021/jacs.3c02447 Leyu Liu 1 , Hai Xiao 1
Affiliation
|
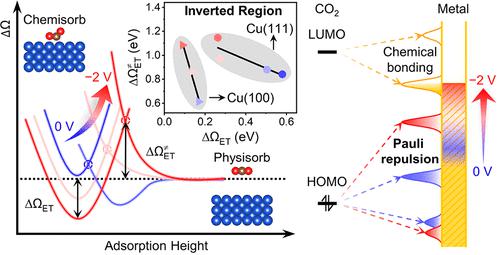
Electrochemical CO2 reduction reaction (eCO2RR) is of great significance to energy and environmental engineering, while fundamental questions remain regarding its mechanisms. Herein, we formulate a fundamental understanding of the interplay between the applied potential (U) and kinetics of CO2 activation in eCO2RR on Cu surfaces. We find that the nature of the CO2 activation mechanism in eCO2RR varies with U, and it is the sequential electron–proton transfer (SEPT) mechanism dominant at the working U but switched to the concerted proton–electron transfer (CPET) mechanism at highly negative U. We then identify that the barrier of the electron-transfer step in the SEPT mechanism exhibits an inverted region as U decreases, which originates from the rapidly rising Pauli repulsion in the physisorption of CO2 with decreasing U. We further demonstrate catalyst designs that effectively suppress the adverse effect of Pauli repulsion. This fundamental understanding may be general for the electrochemical reduction reactions of closed-shell molecules.
中文翻译:

电势相关泡利排斥引起的 CO2 电化学还原中的反转区域
电化学CO 2还原反应(eCO 2 RR)对于能源和环境工程具有重要意义,但其机理仍存在根本问题。在此,我们对铜表面eCO 2 RR 中施加的电势 ( U ) 和 CO 2活化动力学之间的相互作用有了基本的了解。我们发现eCO 2 RR中CO 2活化机制的性质随U的变化而变化,并且在工作U时是顺序电子-质子转移(SEPT)机制占主导地位,但切换到协同质子-电子转移(CPET)机制在高度负U。然后我们发现SEPT机制中电子转移步骤的势垒随着U的减小而呈现出反转区域,这源于随着U的减小CO 2物理吸附中的泡利排斥力迅速上升。我们进一步证明了催化剂设计可以有效抑制泡利排斥的不利影响。这种基本理解对于闭壳分子的电化学还原反应可能是普遍的。
更新日期:2023-06-09
中文翻译:

电势相关泡利排斥引起的 CO2 电化学还原中的反转区域
电化学CO 2还原反应(eCO 2 RR)对于能源和环境工程具有重要意义,但其机理仍存在根本问题。在此,我们对铜表面eCO 2 RR 中施加的电势 ( U ) 和 CO 2活化动力学之间的相互作用有了基本的了解。我们发现eCO 2 RR中CO 2活化机制的性质随U的变化而变化,并且在工作U时是顺序电子-质子转移(SEPT)机制占主导地位,但切换到协同质子-电子转移(CPET)机制在高度负U。然后我们发现SEPT机制中电子转移步骤的势垒随着U的减小而呈现出反转区域,这源于随着U的减小CO 2物理吸附中的泡利排斥力迅速上升。我们进一步证明了催化剂设计可以有效抑制泡利排斥的不利影响。这种基本理解对于闭壳分子的电化学还原反应可能是普遍的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号