当前位置:
X-MOL 学术
›
J. Agric. Food Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Potential Succinate Dehydrogenase Inhibitors Bearing a Novel Pyrazole-4-sulfonohydrazide Scaffold: Molecular Design, Antifungal Evaluation, and Action Mechanism
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2023-06-09 , DOI: 10.1021/acs.jafc.3c00126 Jian-Qi Chai 1, 2 , Yu-Dong Mei 1, 3 , Lang Tai 1 , Xiao-Bin Wang 1, 2, 4 , Min Chen 1, 2 , Xiang-Yi Kong 1 , Ai-Min Lu 1, 2 , Guo-Hua Li 1, 2 , Chun-Long Yang 1, 2
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2023-06-09 , DOI: 10.1021/acs.jafc.3c00126 Jian-Qi Chai 1, 2 , Yu-Dong Mei 1, 3 , Lang Tai 1 , Xiao-Bin Wang 1, 2, 4 , Min Chen 1, 2 , Xiang-Yi Kong 1 , Ai-Min Lu 1, 2 , Guo-Hua Li 1, 2 , Chun-Long Yang 1, 2
Affiliation
|
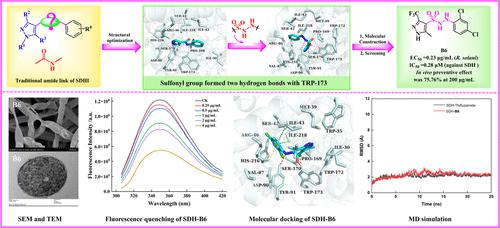
Aiming to develop novel antifungal agents with a distinctive molecular scaffold targeting succinate dehydrogenase (SDH), 24 N′-phenyl-1H-pyrazole-4-sulfonohydrazide derivatives were first devised, synthesized, and verified by 1H NMR, 13C NMR, high-resolution mass spectrometry (HRMS), and single-crystal X-ray diffraction analysis. The bioassays revealed that the target compounds possessed highly efficient and broad-spectrum antifungal activities against four tested plant pathogenic fungi Rhizoctonia solani (R. solani), Botrytis cinerea, Fusarium graminearum, and Alternaria sonali. Strikingly, compound B6 was assessed as the selective inhibitor against R. solani, with an in vitro EC50 value (0.23 μg/mL) that was similar to that of thifluzamide (0.20 μg/mL). The in vivo preventative effect of compound B6 (75.76%) at 200 μg/mL against R. solani was roughly comparable to thifluzamide (84.31%) under the same conditions. The exploration of morphological observations indicated that compound B6 could strongly damage the mycelium morphology, obviously increase the permeability of the cell membrane, and dramatically increase the number of mitochondria. Compound B6 also significantly inhibited SDH enzyme activity with an IC50 value of 0.28 μg/mL, and its fluorescence quenching dynamic curves were similar to that of thifluzamide. Molecular docking and molecular dynamics simulations demonstrated that compound B6 could strongly interact with similar residues around the SDH active pocket as thifluzamide. The present study revealed that the novel N′-phenyl-1H-pyrazole pyrazole-4-sulfonohydrazide derivatives are worthy of being further investigated as the promising replacements of traditional carboxamide derivatives targeting SDH of fungi.
中文翻译:

具有新型吡唑-4-磺酰肼支架的潜在琥珀酸脱氢酶抑制剂:分子设计、抗真菌评价和作用机制
为了开发具有靶向琥珀酸脱氢酶(SDH)的独特分子支架的新型抗真菌剂,首先设计、合成了24 N'-苯基-1 H-吡唑-4-磺酰肼衍生物,并通过1 H NMR、13 C NMR 验证,高分辨率质谱(HRMS)和单晶X射线衍射分析。生物测定结果表明,目标化合物对四种供试植物病原真菌立枯丝核菌(R. solani)、灰葡萄孢、禾谷镰刀菌和黑链格孢具有高效、广谱的抗真菌活性。引人注目的是,复合B6被评估为抗立枯丝核菌的选择性抑制剂,其体外EC 50值 (0.23 μg/mL) 与 thifluzamide (0.20 μg/mL) 相似。相同条件下,200 μg/mL的化合物B6(75.76%)对立枯丝核菌的体内预防效果与thifluzamide(84.31%)大致相当。形态学观察探索表明,化合物B6能强烈破坏菌丝体形态,明显增加细胞膜的通透性,并显着增加线粒体数量。化合物B6还通过 IC 显着抑制 SDH 酶活性50值为0.28 μg/mL,其荧光猝灭动态曲线与thifluzamide相似。分子对接和分子动力学模拟表明,化合物B6可以与 SDH 活性口袋周围的类似残基(如 thifluzamide)强烈相互作用。本研究表明,新型N'-苯基-1H-吡唑吡唑-4-磺酰肼衍生物作为针对真菌SDH的传统甲酰胺衍生物的有前景的替代品值得进一步研究。
更新日期:2023-06-09
中文翻译:

具有新型吡唑-4-磺酰肼支架的潜在琥珀酸脱氢酶抑制剂:分子设计、抗真菌评价和作用机制
为了开发具有靶向琥珀酸脱氢酶(SDH)的独特分子支架的新型抗真菌剂,首先设计、合成了24 N'-苯基-1 H-吡唑-4-磺酰肼衍生物,并通过1 H NMR、13 C NMR 验证,高分辨率质谱(HRMS)和单晶X射线衍射分析。生物测定结果表明,目标化合物对四种供试植物病原真菌立枯丝核菌(R. solani)、灰葡萄孢、禾谷镰刀菌和黑链格孢具有高效、广谱的抗真菌活性。引人注目的是,复合B6被评估为抗立枯丝核菌的选择性抑制剂,其体外EC 50值 (0.23 μg/mL) 与 thifluzamide (0.20 μg/mL) 相似。相同条件下,200 μg/mL的化合物B6(75.76%)对立枯丝核菌的体内预防效果与thifluzamide(84.31%)大致相当。形态学观察探索表明,化合物B6能强烈破坏菌丝体形态,明显增加细胞膜的通透性,并显着增加线粒体数量。化合物B6还通过 IC 显着抑制 SDH 酶活性50值为0.28 μg/mL,其荧光猝灭动态曲线与thifluzamide相似。分子对接和分子动力学模拟表明,化合物B6可以与 SDH 活性口袋周围的类似残基(如 thifluzamide)强烈相互作用。本研究表明,新型N'-苯基-1H-吡唑吡唑-4-磺酰肼衍生物作为针对真菌SDH的传统甲酰胺衍生物的有前景的替代品值得进一步研究。











































 京公网安备 11010802027423号
京公网安备 11010802027423号