当前位置:
X-MOL 学术
›
J. Agric. Food Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
IgE Epitope Analysis and Hypo-Immunoreactivity Derivative of Arginine Kinase in Mantis Shrimp (Oratosquilla oratoria)
Journal of Agricultural and Food Chemistry ( IF 6.1 ) Pub Date : 2023-06-08 , DOI: 10.1021/acs.jafc.3c01549 Ye-Xin Chen 1 , Xin-Rong He 1 , Shi-Qiang Yang 1 , Fei Huan 1 , Dong-Xiao Li 1 , Yang Yang 1, 2 , Gui-Xia Chen 3 , Guang-Ming Liu 1
Journal of Agricultural and Food Chemistry ( IF 6.1 ) Pub Date : 2023-06-08 , DOI: 10.1021/acs.jafc.3c01549 Ye-Xin Chen 1 , Xin-Rong He 1 , Shi-Qiang Yang 1 , Fei Huan 1 , Dong-Xiao Li 1 , Yang Yang 1, 2 , Gui-Xia Chen 3 , Guang-Ming Liu 1
Affiliation
|
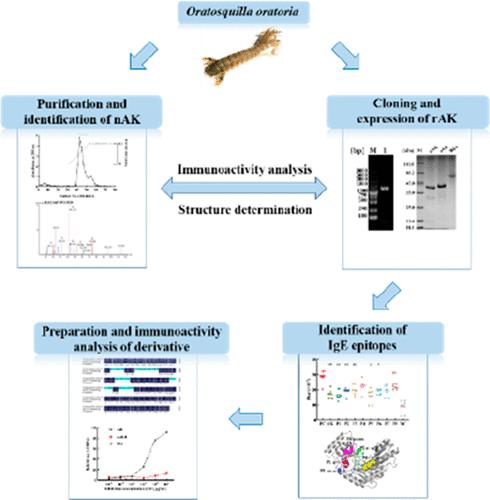
As the main allergenic food, shrimp can trigger allergic reactions in various degrees. In this study, arginine kinase (AK) was identified as an allergen in Oratosquilla oratoria by LC–MS/MS. The open reading frame of AK was obtained, which included 356 amino acids, and recombinant AK (rAK) was expressed in Escherichia coli. The results of immunological analysis and circular dichroism showed that rAK displayed similar IgG-/IgE-binding activity and structure as native AK. Besides, five IgE linear epitopes of AK were verified by serological analysis, on the basis of which an epitope-deleted derivative was obtained and named as mAK-L. It has been shown that mAK-L displayed hypo-immunoreactivity compared to rAK, and the contents of secondary structures were different. In conclusion, these discoveries enrich the overall understanding of crustacean allergens and epitopes and set the foundations for food allergy diagnosis and immunotherapy.
中文翻译:

螳螂虾(口虾蛄)精氨酸激酶的 IgE 表位分析和低免疫反应性衍生物
虾作为主要的致敏食品,可引发不同程度的过敏反应。在本研究中,精氨酸激酶 (AK) 通过 LC-MS/MS 被鉴定为口虾蛄中的过敏原。获得AK的开放阅读框,包含356个氨基酸,并在大肠杆菌中表达重组AK(rAK)。免疫学分析和圆二色性结果表明,rAK 表现出与天然 AK 相似的 IgG-/IgE 结合活性和结构。此外,通过血清学分析验证了AK的5个IgE线性表位,在此基础上得到表位缺失的衍生物,命名为mAK-L。研究表明,与rAK相比,mAK-L的免疫反应性较低,并且二级结构的含量不同。总之,这些发现丰富了对甲壳类过敏原和表位的整体理解,并为食物过敏诊断和免疫治疗奠定了基础。
更新日期:2023-06-08
中文翻译:

螳螂虾(口虾蛄)精氨酸激酶的 IgE 表位分析和低免疫反应性衍生物
虾作为主要的致敏食品,可引发不同程度的过敏反应。在本研究中,精氨酸激酶 (AK) 通过 LC-MS/MS 被鉴定为口虾蛄中的过敏原。获得AK的开放阅读框,包含356个氨基酸,并在大肠杆菌中表达重组AK(rAK)。免疫学分析和圆二色性结果表明,rAK 表现出与天然 AK 相似的 IgG-/IgE 结合活性和结构。此外,通过血清学分析验证了AK的5个IgE线性表位,在此基础上得到表位缺失的衍生物,命名为mAK-L。研究表明,与rAK相比,mAK-L的免疫反应性较低,并且二级结构的含量不同。总之,这些发现丰富了对甲壳类过敏原和表位的整体理解,并为食物过敏诊断和免疫治疗奠定了基础。



























 京公网安备 11010802027423号
京公网安备 11010802027423号