当前位置:
X-MOL 学术
›
Ind. Eng. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Analysis of the Composition and Formation Mechanism of Fouling in the Concentration Process of Titanium White Waste Acid
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2023-06-01 , DOI: 10.1021/acs.iecr.3c00951 Bowen Xu 1 , Tao Zhang 1 , Li Lv 1 , Wenxiang Tang 1 , Yan Wang 1 , Jiabei Zhou 1 , Shengwei Tang 1
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2023-06-01 , DOI: 10.1021/acs.iecr.3c00951 Bowen Xu 1 , Tao Zhang 1 , Li Lv 1 , Wenxiang Tang 1 , Yan Wang 1 , Jiabei Zhou 1 , Shengwei Tang 1
Affiliation
|
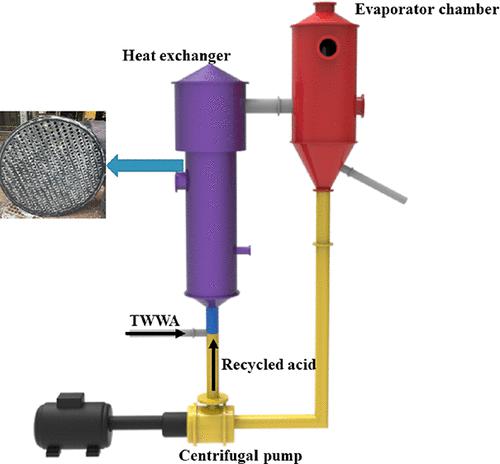
Large quantities of titanium white waste acid (TWWA) are generated during TiO2 production by the sulfate process. Recycling by concentration is the most widely used method to treat TWWA. However, serious fouling occurs during this process. The chemical composition of fouling obtained from a commercial plant was analyzed. The phase equilibria of CaSO4-FeSO4-H2SO4-H2O at 373.15 and 378.15 K were studied to elucidate the fouling mechanism. The fouling was mainly composed of CaSO4 (anhydrite) (74.97 wt %) and TiO(OH)2 (anatase) (24.48 wt %). The mass fractions of CaSO4 and FeSO4 in the equilibrium liquid phase of the CaSO4-FeSO4-H2SO4-H2O system increased with an increase in temperature and a decrease in H2SO4 concentration. The Pitzer method was successfully employed to describe the equilibrium behavior. During the mixing of the recycled acid and the initial TWWA, some CaSO4 (anhydrite) precipitated from the liquid phase, while some FeSO4·H2O dissolved in the liquid phase during the mixing and heating process. In the heating process, CaSO4 (anhydrite) did not precipitate out from the liquid phase. It was thus concluded that CaSO4 (anhydrite) precipitation during the mixing process is the key factor for the fouling on the heat exchanger surface during the primary evaporation process.
中文翻译:

钛白废酸浓缩过程结垢成分及形成机理分析
硫酸法生产TiO 2过程中会产生大量的钛白废酸(TWWA) 。浓缩回收是处理TWWA最广泛使用的方法。然而,在此过程中会发生严重的结垢。分析了从商业工厂获得的污垢的化学成分。研究了 CaSO 4 -FeSO 4 -H 2 SO 4 -H 2 O 在 373.15 和 378.15 K的相平衡以阐明结垢机理。污垢主要由 CaSO 4 (硬石膏) (74.97 wt%) 和 TiO(OH) 2 (锐钛矿) (24.48 wt%) 组成。CaSO 4和FeSO 4的质量分数在CaSO 4 -FeSO 4 -H 2 SO 4 -H 2 O体系的平衡液相中,随着温度的升高和H 2 SO 4浓度的降低而升高。Pitzer 方法成功地用于描述平衡行为。在回收酸和初始TWWA混合过程中,一些CaSO 4 (硬石膏)从液相中析出,而一些FeSO 4 ·H 2 O在混合和加热过程中溶解在液相中。在加热过程中,CaSO 4(无水石膏)没有从液相中沉淀出来。由此得出结论,混合过程中CaSO 4(硬石膏)沉淀是一次蒸发过程中换热器表面结垢的关键因素。
更新日期:2023-06-01
中文翻译:

钛白废酸浓缩过程结垢成分及形成机理分析
硫酸法生产TiO 2过程中会产生大量的钛白废酸(TWWA) 。浓缩回收是处理TWWA最广泛使用的方法。然而,在此过程中会发生严重的结垢。分析了从商业工厂获得的污垢的化学成分。研究了 CaSO 4 -FeSO 4 -H 2 SO 4 -H 2 O 在 373.15 和 378.15 K的相平衡以阐明结垢机理。污垢主要由 CaSO 4 (硬石膏) (74.97 wt%) 和 TiO(OH) 2 (锐钛矿) (24.48 wt%) 组成。CaSO 4和FeSO 4的质量分数在CaSO 4 -FeSO 4 -H 2 SO 4 -H 2 O体系的平衡液相中,随着温度的升高和H 2 SO 4浓度的降低而升高。Pitzer 方法成功地用于描述平衡行为。在回收酸和初始TWWA混合过程中,一些CaSO 4 (硬石膏)从液相中析出,而一些FeSO 4 ·H 2 O在混合和加热过程中溶解在液相中。在加热过程中,CaSO 4(无水石膏)没有从液相中沉淀出来。由此得出结论,混合过程中CaSO 4(硬石膏)沉淀是一次蒸发过程中换热器表面结垢的关键因素。











































 京公网安备 11010802027423号
京公网安备 11010802027423号