Water Research ( IF 11.4 ) Pub Date : 2023-05-26 , DOI: 10.1016/j.watres.2023.120133 Fu-Sheng Sun 1 , Chao Ma 1 , Guang-Hui Yu 1 , Yakov Kuzyakov 2 , Yun-Chao Lang 1 , Ping-Qing Fu 1 , Li-Jun Guo 3 , Hui Henry Teng 4 , Cong-Qiang Liu 1
|
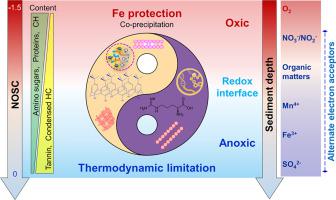
The sequestration of organic carbon (OC) in wetland sediments is influenced by the presence of oxygen or lack thereof. The mechanisms of OC sequestration under redox fluctuations, particularly by the co-mediation of reactive iron (Fe) protection and thermodynamic limitation by the energetics of the OC itself, remain unclear. Over the past 26 years, a combination of field surveys and remote sensing images had revealed a strong decline in both natural and constructed wetland areas in Tianjin. This decline could be attributed to anthropogenic landfill practices and agricultural reclamation efforts, which may have significant impacts on the oxidation–reduction conditions for sedimentary OC. The Fe-bound OC (CBD extraction) decreased by 2 to 10-fold (from 8.3 to 10% to 0.7–4.5%) with increasing sediment depth at three sites with varying water depths (WD). The high-resolution spectro-microscopy analysis demonstrated that Fe (oxyhydr)oxides were colocalized with sedimentary OC. Corresponding to lower redox potential, the nominal oxidation state of C (NOSC), which corresponds to the energy content in OC, became more negative (energy content increased) with increasing sediment depth. Taken together, the preservation of sedimentary OC is contingent on the prevailing redox conditions: In environments where oxygen availability is high, reactive Fe provides protection for OC, while in anoxic environments, thermodynamic constraints (i.e., energetic constraints) limit the oxidation of OC.
中文翻译:

湿地中的有机碳保存:氧化铁保护与热力学限制
湿地沉积物中有机碳(OC)的封存受到氧气存在或缺乏的影响。氧化还原波动下 OC 封存的机制,特别是通过活性铁 (Fe) 保护和 OC 本身能量学的热力学限制的共同介导,仍不清楚。过去26年来,结合实地调查和遥感图像显示,天津的自然湿地和人工湿地面积均大幅减少。这种下降可能归因于人为垃圾填埋做法和农业复垦工作,这可能对沉积有机碳的氧化还原条件产生重大影响。Fe 结合的 OC(CBD 提取)减少了 2 至 10 倍(从 8.3% 至 10% 降至 0.7-4%)。5%),随着三个水深(WD)不同的地点沉积物深度的增加。高分辨率光谱显微镜分析表明,Fe(羟基)氧化物与沉积物 OC 共存。对应于较低的氧化还原电位,C的标称氧化态(NOSC)(对应于OC中的能量含量)随着沉积物深度的增加变得更加负(能量含量增加)。总而言之,沉积物 OC 的保存取决于主要的氧化还原条件:在氧气利用率高的环境中,活性 Fe 为 OC 提供保护,而在缺氧环境中,热力学约束(即能量约束)限制 OC 的氧化。对应于较低的氧化还原电位,C的标称氧化态(NOSC)(对应于OC中的能量含量)随着沉积物深度的增加变得更加负(能量含量增加)。总而言之,沉积物 OC 的保存取决于主要的氧化还原条件:在氧气利用率高的环境中,活性 Fe 为 OC 提供保护,而在缺氧环境中,热力学约束(即能量约束)限制 OC 的氧化。对应于较低的氧化还原电位,C的标称氧化态(NOSC)(对应于OC中的能量含量)随着沉积物深度的增加变得更加负(能量含量增加)。总而言之,沉积物 OC 的保存取决于主要的氧化还原条件:在氧气利用率高的环境中,活性 Fe 为 OC 提供保护,而在缺氧环境中,热力学约束(即能量约束)限制 OC 的氧化。










































 京公网安备 11010802027423号
京公网安备 11010802027423号