当前位置:
X-MOL 学术
›
Eur. J. Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Insight into Solvent Coordination of An Iron Porphyrin Hydroxylamine Complex from 1H NMR, FTIR and DFT Evidence
European Journal of Inorganic Chemistry ( IF 2.2 ) Pub Date : 2018-03-25 , DOI: 10.1002/ejic.201800040 Md. Hafizur Rahman 1 , Michael D. Ryan 1
European Journal of Inorganic Chemistry ( IF 2.2 ) Pub Date : 2018-03-25 , DOI: 10.1002/ejic.201800040 Md. Hafizur Rahman 1 , Michael D. Ryan 1
Affiliation
|
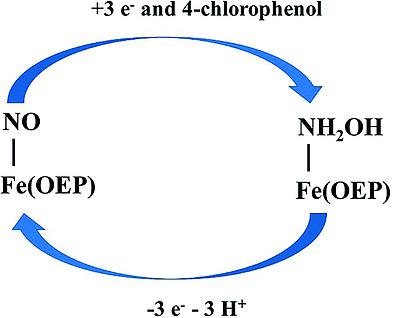
The reduction of Fe(OEP)(NO) in the presence of substituted phenols leads to a three‐electron reduction to form Fe(OEP)(NH2OH), which has been characterized by visible spectroscopy and electron stoichiometry. In this work, we have further characterized this species using infrared and H NMR spectroscopy, along with DFT calculations. The infrared bands in the 3400–3600 cm region, due to hydroxylamine, were significantly downshifted to the 2500–2700 cm region when 4‐[D1]chlorophenol replaced the normal abundance acid. Using H NMR spectroscopy, the hydroxylamine and the meso‐protons were identified. From DFT calculations, the H NMR spectra were most consistent with a six‐coordinate complex, Fe(OEP)(NH2OH)(THF).
中文翻译:

从 1H NMR、FTIR 和 DFT 证据洞察铁卟啉羟胺配合物的溶剂配位
在取代酚的存在下,Fe(OEP)(NO)的还原导致三电子还原形成 Fe(OEP)(NH2OH),其特征在于可见光谱和电子化学计量学。在这项工作中,我们使用红外和 H NMR 光谱以及 DFT 计算进一步表征了该物种。当 4-[D1] 氯苯酚取代正常丰度酸时,由于羟胺,3400-3600 cm 区域的红外波段显着下移至 2500-2700 cm 区域。使用 H NMR 光谱,确定了羟胺和中质子。根据 DFT 计算,H NMR 光谱与六配位络合物 Fe(OEP)(NH2OH)(THF) 最一致。
更新日期:2018-03-25
中文翻译:

从 1H NMR、FTIR 和 DFT 证据洞察铁卟啉羟胺配合物的溶剂配位
在取代酚的存在下,Fe(OEP)(NO)的还原导致三电子还原形成 Fe(OEP)(NH2OH),其特征在于可见光谱和电子化学计量学。在这项工作中,我们使用红外和 H NMR 光谱以及 DFT 计算进一步表征了该物种。当 4-[D1] 氯苯酚取代正常丰度酸时,由于羟胺,3400-3600 cm 区域的红外波段显着下移至 2500-2700 cm 区域。使用 H NMR 光谱,确定了羟胺和中质子。根据 DFT 计算,H NMR 光谱与六配位络合物 Fe(OEP)(NH2OH)(THF) 最一致。







































 京公网安备 11010802027423号
京公网安备 11010802027423号