当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
A novel sulfonamide non-classical carbenoid: a mechanistic study for the synthesis of enediynes†
Organic & Biomolecular Chemistry ( IF 3.2 ) Pub Date : 2017-11-20 00:00:00 , DOI: 10.1039/c7ob02437a Theodore O. P. Hayes 1, 2, 3, 4 , Ben Slater 1, 2, 3, 4 , Richard A. J. Horan 4, 5, 6, 7 , Marc Radigois 1, 2, 3, 4 , Jonathan D. Wilden 1, 2, 3, 4
Organic & Biomolecular Chemistry ( IF 3.2 ) Pub Date : 2017-11-20 00:00:00 , DOI: 10.1039/c7ob02437a Theodore O. P. Hayes 1, 2, 3, 4 , Ben Slater 1, 2, 3, 4 , Richard A. J. Horan 4, 5, 6, 7 , Marc Radigois 1, 2, 3, 4 , Jonathan D. Wilden 1, 2, 3, 4
Affiliation
|
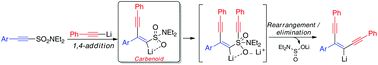
Alkynyl sulfonamides undergo sequential 1,4- then 1,2-addition/rearrangement with lithium acetylides to yield enediynes in the absence of any promoters or catalysts. Mechanistic investigations suggest that the reaction proceeds via 1,4-conjugate addition of the nucleophile to the unsaturated system to give a key alkenyl lithium species which is stabilised by an intramolecular coordination effect by a sulfonamide oxygen atom. This species can be considered a vinylidene carbenoid given the carbon atom bears both an anion (as a vinyllithium) and a leaving group (the sulfonamide). The intramolecular coordination effect serves to stabilise the vinyllithium but activates the sulfonamide motif towards nucleophilic attack by a second mole of acetylide. The resulting species can then undergo rearrangement to yield the enediyne framework in a single operation with concomitant loss of aminosulfinate.
中文翻译:

新型磺酰胺类非经典类胡萝卜素:烯二炔合成的机理研究†
在没有任何促进剂或催化剂的情况下,炔基磺酰胺与乙炔锂依次进行1,4-然后1,2-加成/重排,生成烯二炔。机理研究表明,反应通过亲核试剂的1,4-共轭加成到不饱和系统上,从而得到关键的烯基锂物质,该物质通过磺酰胺氧原子的分子内配位作用得以稳定。考虑到碳原子既带有阴离子(作为乙烯基锂)又带有离去基团(磺酰胺),该物种可以被视为亚乙烯基类化合物。分子内配位作用起到稳定乙烯基锂的作用,但激活磺酰胺基序,使其受到第二摩尔乙炔化物的亲核攻击。然后,所产生的物质可以在单个操作中进行重排以产生烯二炔构架,同时伴随着氨基亚磺酸盐的损失。
更新日期:2017-11-20
中文翻译:

新型磺酰胺类非经典类胡萝卜素:烯二炔合成的机理研究†
在没有任何促进剂或催化剂的情况下,炔基磺酰胺与乙炔锂依次进行1,4-然后1,2-加成/重排,生成烯二炔。机理研究表明,反应通过亲核试剂的1,4-共轭加成到不饱和系统上,从而得到关键的烯基锂物质,该物质通过磺酰胺氧原子的分子内配位作用得以稳定。考虑到碳原子既带有阴离子(作为乙烯基锂)又带有离去基团(磺酰胺),该物种可以被视为亚乙烯基类化合物。分子内配位作用起到稳定乙烯基锂的作用,但激活磺酰胺基序,使其受到第二摩尔乙炔化物的亲核攻击。然后,所产生的物质可以在单个操作中进行重排以产生烯二炔构架,同时伴随着氨基亚磺酸盐的损失。



























 京公网安备 11010802027423号
京公网安备 11010802027423号