Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of highly fluorescent gold nanoclusters and their use in sensitive analysis of metal ions
Analyst ( IF 3.6 ) Pub Date : 2017-10-17 00:00:00 , DOI: 10.1039/c7an01348e Yayu Yang 1, 2, 3, 4, 5 , Ailing Han 1, 2, 3, 4, 5 , Ruixue Li 1, 2, 3, 4, 5 , Guozhen Fang 1, 2, 3, 4, 5 , Jifeng Liu 1, 2, 3, 4, 5 , Shuo Wang 1, 2, 3, 4, 5
Analyst ( IF 3.6 ) Pub Date : 2017-10-17 00:00:00 , DOI: 10.1039/c7an01348e Yayu Yang 1, 2, 3, 4, 5 , Ailing Han 1, 2, 3, 4, 5 , Ruixue Li 1, 2, 3, 4, 5 , Guozhen Fang 1, 2, 3, 4, 5 , Jifeng Liu 1, 2, 3, 4, 5 , Shuo Wang 1, 2, 3, 4, 5
Affiliation
|
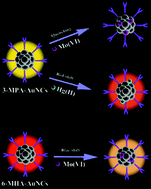
As a promising fluorescent material, gold nanoclusters (Au NCs) have been used for biosensing and imaging. In metal ion sensing, the fluorescence of Au NCs is usually quenched in the presence of ions, but the reaction mechanism has not been clarified. In this work, mercaptan acids (3-mercaptopropanoic acid (3-MPA), 6-mercaptohexanoic acid (6-MHA), 11-mercaptoundecanoic acid (11-MUA) and 16-mercaptohexadecanoic acid (16-MHA)) were used as reductants and ligands to synthesize fluorescent Au NCs. The Au NCs had a core containing 18 atoms capped by 18 (3-MPA) or 10 (6-MHA, 11-MUA, 16-MHA) ligand molecules. The Au NCs prepared in this work had higher fluorescence quantum yields than those reported previously and the fluorescence showed a red-shift when varying the chain length of the mercaptan acids. It was found that in presence of ions (Mo(VI), Hg(II)), the fluorescence of the Au NCs is dependent upon the ligands. The Au NCs with 3-MPA and 6-MHA ligands were sensitive to Mo(VI) and Hg(II), while the Au NCs capped with 11-MUA and 16-MHA ligands were not sensitive to the ions. Various characterization techniques, including transmission electron microscopy, matrix-assisted laser-desorption ionization time of flight mass spectrometry, X-ray photoelectron spectroscopy, UV-Vis absorption spectroscopy and thermogravimetric analysis, were used to investigate the components and electronic structure of the clusters, as well as the emission and excitation properties before and after the reaction with metal ions. The structure of the Au NCs after the reaction with Mo(VI) and Hg(II) was also studied. It was found that Mo(VI) and Hg(II) both reacted with the gold atoms at the core site, but the reaction mechanisms were different.
中文翻译:

高荧光金纳米团簇的合成及其在金属离子灵敏分析中的应用
作为一种有前途的荧光材料,金纳米团簇(Au NCs)已用于生物传感和成像。在金属离子感测中,Au NCs的荧光通常在离子存在下被淬灭,但反应机理尚未阐明。在这项工作中,硫醇酸(3-巯基丙酸(3-MPA),6-巯基己酸(6-MHA),11-巯基十一酸(11-MUA)和16-巯基十六酸(16-MHA))被用作还原剂和配体来合成荧光Au NCs。Au NCs具有一个包含18个原子的核,该18个原子被18个(3-MPA)或10个(6-MHA,11-MUA,16-MHA)配体分子封端。这项工作中制备的Au NCs的荧光量子产率比以前报道的要高,并且当改变硫醇酸的链长时,荧光显示出红移。发现存在离子(Mo(VI),Hg(II)),Au NCs的荧光取决于配体。具有3-MPA和6-MHA配体的Au NCs对Mo(VI)和Hg(II)敏感,而具有11-MUA和16-MHA配体的Au NCs对离子不敏感。包括透射电子显微镜,基质辅助激光解吸电离飞行时间质谱,X射线光电子能谱,UV-Vis吸收能谱和热重分析在内的各种表征技术用于研究团簇的组成和电子结构,与金属离子反应之前和之后的发射和激发特性。与Mo(VI)反应后的Au NCs的结构)和Hg(II)也进行了研究。发现Mo(VI)和Hg(II)都与核心位点的金原子反应,但反应机理不同。
更新日期:2017-11-20
中文翻译:

高荧光金纳米团簇的合成及其在金属离子灵敏分析中的应用
作为一种有前途的荧光材料,金纳米团簇(Au NCs)已用于生物传感和成像。在金属离子感测中,Au NCs的荧光通常在离子存在下被淬灭,但反应机理尚未阐明。在这项工作中,硫醇酸(3-巯基丙酸(3-MPA),6-巯基己酸(6-MHA),11-巯基十一酸(11-MUA)和16-巯基十六酸(16-MHA))被用作还原剂和配体来合成荧光Au NCs。Au NCs具有一个包含18个原子的核,该18个原子被18个(3-MPA)或10个(6-MHA,11-MUA,16-MHA)配体分子封端。这项工作中制备的Au NCs的荧光量子产率比以前报道的要高,并且当改变硫醇酸的链长时,荧光显示出红移。发现存在离子(Mo(VI),Hg(II)),Au NCs的荧光取决于配体。具有3-MPA和6-MHA配体的Au NCs对Mo(VI)和Hg(II)敏感,而具有11-MUA和16-MHA配体的Au NCs对离子不敏感。包括透射电子显微镜,基质辅助激光解吸电离飞行时间质谱,X射线光电子能谱,UV-Vis吸收能谱和热重分析在内的各种表征技术用于研究团簇的组成和电子结构,与金属离子反应之前和之后的发射和激发特性。与Mo(VI)反应后的Au NCs的结构)和Hg(II)也进行了研究。发现Mo(VI)和Hg(II)都与核心位点的金原子反应,但反应机理不同。











































 京公网安备 11010802027423号
京公网安备 11010802027423号