当前位置:
X-MOL 学术
›
Chem. Eur. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Reducing Diastereomorphous Bis(phosphane oxide) Atropisomers to One Atropisomerically Pure Diphosphane: A New Ligand and a Novel Ligand‐Preparation Design
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2017-11-20 , DOI: 10.1002/chem.201704800 Frank Sartorius 1, 2 , Marc Trebing 1, 3 , Charlotte Brückner 4 , Reinhard Brückner 1
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2017-11-20 , DOI: 10.1002/chem.201704800 Frank Sartorius 1, 2 , Marc Trebing 1, 3 , Charlotte Brückner 4 , Reinhard Brückner 1
Affiliation
|
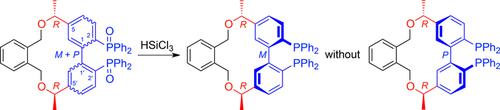
1,1′‐Biphenyl‐2,2′‐diphosphanes with an achiral bridge spanning C‐5 and C‐5′ form atropisomers that are enantiomers. Accessing them in an atropisomerically pure form requires resolving a racemic mixture thereof or of a bis(phosphane oxide) precursor. 1,1′‐Biphenyl‐2,2′‐diphosphanes with a homochiral bridge spanning C‐5 and C‐5′ form atropisomers that are diastereomers. We synthesized the first compound of this kind 1) atropselectively and 2) under thermodynamic control—seemingly a first‐time exploit in diphosphane synthesis. The selectivity‐inducing step was a high‐temperature reduction of two non‐interconverting bis(phosphane oxide) atropisomers (60:40 mixture). It furnished the desired diphosphane atropisomerically pure (and atropconvergently because the yield was 67 %). This diphosphane proved worthwhile in Tsuji–Trost allylations, the Hayashi addition of phenylboronic acid to cyclohexenone, and the asymmetric hydrogenation of methyl acetoacetate (up to 95 % yield and 95 % ee).
中文翻译:

还原非对映体的双(氧化膦)阻转异构体为一种非对映异构纯二膦:一种新的配体和一种新颖的配体制备设计
具有跨越C-5和C-5'的非手性桥的1,1'-联苯-2,2'-二膦烷形成为对映异构体的阻转异构体。以对映异构纯的形式获得它们需要解析其外消旋混合物或双(氧化膦)前体的外消旋混合物。具有跨越C-5和C-5'的同手性桥的1,1'-联苯-2,2'-二膦形成非对映异构体的阻转异构体。我们合成了第一种化合物1)选择性地和2)在热力学控制下—似乎是二膦合成中的首次利用。诱导选择性的步骤是高温还原两种非相互转化的双(氧化膦)阻转异构体(60:40的混合物)。它提供了所需的二膦烷非对映异构纯的(并且由于聚合产率为67%,因此不易对流)。在Tsuji-Trost烯丙基化反应中,这种二膦烷被证明是值得的,ee)。
更新日期:2017-11-20
中文翻译:

还原非对映体的双(氧化膦)阻转异构体为一种非对映异构纯二膦:一种新的配体和一种新颖的配体制备设计
具有跨越C-5和C-5'的非手性桥的1,1'-联苯-2,2'-二膦烷形成为对映异构体的阻转异构体。以对映异构纯的形式获得它们需要解析其外消旋混合物或双(氧化膦)前体的外消旋混合物。具有跨越C-5和C-5'的同手性桥的1,1'-联苯-2,2'-二膦形成非对映异构体的阻转异构体。我们合成了第一种化合物1)选择性地和2)在热力学控制下—似乎是二膦合成中的首次利用。诱导选择性的步骤是高温还原两种非相互转化的双(氧化膦)阻转异构体(60:40的混合物)。它提供了所需的二膦烷非对映异构纯的(并且由于聚合产率为67%,因此不易对流)。在Tsuji-Trost烯丙基化反应中,这种二膦烷被证明是值得的,ee)。











































 京公网安备 11010802027423号
京公网安备 11010802027423号