当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Characterization of Platinum Electrode Surfaces by Electrochemical Surface Forces Measurement
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2017-11-20 00:00:00 , DOI: 10.1021/acs.jpcc.7b09301 Sho Fujii 1 , Motohiro Kasuya 1 , Kazue Kurihara 1
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2017-11-20 00:00:00 , DOI: 10.1021/acs.jpcc.7b09301 Sho Fujii 1 , Motohiro Kasuya 1 , Kazue Kurihara 1
Affiliation
|
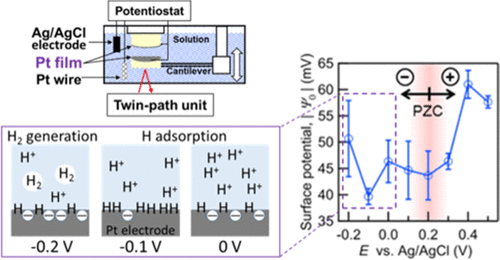
The surface forces between platinum, Pt, electrodes and those between the Pt electrode and mica in aqueous HClO4 were measured at various potentials (E) applied to the electrodes using an electrochemical surface forces apparatus (EC-SFA). This apparatus uses the twin-path surface forces apparatus, recently developed for opaque samples. The influence of the proton adsorption on the surface interactions was studied. The Pt electrodes were prepared by the template-stripping procedure using glass templates. The electrode surfaces were smooth (RMS roughness: 0.26 nm for a 5 μm × 5 μm area) and polycrystalline based on the atomic force microscopy and cyclic voltammetry results, respectively. When the applied potential E was decreased from 0.5 to 0.2 V (vs Ag/AgCl), the electric double layer (EDL) repulsion between the Pt electrodes decreased. The absolute values of the surface potentials, |ψ0|, calculated using the EDL theory were 58 and 43 mV at E = 0.5 and 0.2 V, respectively. The EDL force at E = 0.2 V was the local minimum, suggesting that the potential of the zero charge (PZC) of the Pt electrode was around 0.2 V in the 1 mM HClO4 solution. With the further decreasing potential E from 0.2 to −0.2 V, the EDL repulsion remained similar in amplitude, took another minimum, |ψ0| = 40 mV, at E = −0.1 V, and started to increase again at E = −0.1 V. These behaviors could be caused by proton adsorption on the Pt surface (Ptδ−···H+), the electrochemical hydrogen adsorption (Pt–H), and the subsequent hydrogen evolution (H2↑). The possibility for characterizing the hydrogen evolution processes on the Pt electrodes based on the surface forces measurement is discussed for the first time.
中文翻译:

通过电化学表面力测量表征铂电极表面
使用电化学表面力装置(EC-SFA)在施加到电极上的各种电势(E)下测量了HClO 4水溶液中铂,Pt电极之间以及Pt电极与云母之间的表面力。该设备使用了最近开发的用于不透明样品的双路径表面力设备。研究了质子吸附对表面相互作用的影响。通过使用玻璃模板的模板剥离程序来制备Pt电极。根据原子力显微镜和循环伏安法的结果,电极表面光滑(RMS粗糙度:5μm×5μm区域为0.26 nm)和多晶。当施加电位E时如果将其从0.5 V降低至0.2 V(vs Ag / AgCl),则Pt电极之间的双电层(EDL)排斥力会降低。表面电位的绝对值,|Ψ 0 |,使用EDL理论计算分别为58和43毫伏在Ë = 0.5和0.2分别V,。E = 0.2 V时的EDL力是局部最小值,表明在1 mM HClO 4溶液中Pt电极的零电荷(PZC)电位约为0.2V 。随着进一步降低潜在Ë 0.2〜-0.2 V时,EDL排斥留在幅度相似,又过了最低,|ψ 0 | = 40 mV,在E = −0.1 V时,并在E处再次开始增加= -0.1V。这些行为可能是由于质子吸附在Pt表面(Ptδ ···H +),电化学氢吸附(Pt–H)和随后的氢逸出(H 2 ↑)引起的。首次讨论了基于表面力测量来表征Pt电极上氢析出过程的可能性。
更新日期:2017-11-20
中文翻译:

通过电化学表面力测量表征铂电极表面
使用电化学表面力装置(EC-SFA)在施加到电极上的各种电势(E)下测量了HClO 4水溶液中铂,Pt电极之间以及Pt电极与云母之间的表面力。该设备使用了最近开发的用于不透明样品的双路径表面力设备。研究了质子吸附对表面相互作用的影响。通过使用玻璃模板的模板剥离程序来制备Pt电极。根据原子力显微镜和循环伏安法的结果,电极表面光滑(RMS粗糙度:5μm×5μm区域为0.26 nm)和多晶。当施加电位E时如果将其从0.5 V降低至0.2 V(vs Ag / AgCl),则Pt电极之间的双电层(EDL)排斥力会降低。表面电位的绝对值,|Ψ 0 |,使用EDL理论计算分别为58和43毫伏在Ë = 0.5和0.2分别V,。E = 0.2 V时的EDL力是局部最小值,表明在1 mM HClO 4溶液中Pt电极的零电荷(PZC)电位约为0.2V 。随着进一步降低潜在Ë 0.2〜-0.2 V时,EDL排斥留在幅度相似,又过了最低,|ψ 0 | = 40 mV,在E = −0.1 V时,并在E处再次开始增加= -0.1V。这些行为可能是由于质子吸附在Pt表面(Ptδ ···H +),电化学氢吸附(Pt–H)和随后的氢逸出(H 2 ↑)引起的。首次讨论了基于表面力测量来表征Pt电极上氢析出过程的可能性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号