当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Molecular Level Investigation of CH4 and CO2 Adsorption in Hydrated Calcium–Montmorillonite
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2017-11-17 00:00:00 , DOI: 10.1021/acs.jpcc.7b05364 Mal-Soon Lee 1 , B. Peter McGrail 1 , Roger Rousseau 1 , Vassiliki-Alexandra Glezakou 1
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2017-11-17 00:00:00 , DOI: 10.1021/acs.jpcc.7b05364 Mal-Soon Lee 1 , B. Peter McGrail 1 , Roger Rousseau 1 , Vassiliki-Alexandra Glezakou 1
Affiliation
|
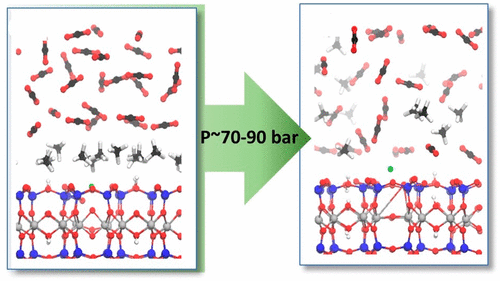
We have studied the mechanism of intercalation and methane adsorption from a H2O/CH4/CO2 mixture on a prototypical swelling shale component, Ca–montmorillonite. We employed ab initio molecular dynamics simulations at 323 K and 90 bar to obtain molecular level information on adsorption energetics, speciation, and structural and thermodynamic properties. Interaction of CH4 with surface Lewis acidic sites (Ca2+, surface OH) results in large induced dipoles (∼1 D) that lead to relatively strong adsorption energies compared to interactions of the normally apolar CH4 that level off once a CH4 layer is formed. Intercalated CH4, also exhibits large induced dipoles at lower hydration levels, when the interaction with Ca2+ cations are less hindered. CO2 displaces CH4 in the coordination sphere of the cations (in the interlayer) or on the surface, thereby driving CH4 extraction. Our simulations indicate that there is an optimal pressure range (∼70–90 bar) where scCO2-facilitated CH4 extraction will be maximized.
中文翻译:

水合钙-蒙脱土中CH 4和CO 2吸附的分子水平研究
我们已经研究了H 2 O / CH 4 / CO 2混合物在典型的膨胀页岩成分Ca–蒙脱石上的嵌入和甲烷吸附的机理。我们在323 K和90 bar的压力下进行了从头算分子动力学模拟,以获取有关吸附能,形态以及结构和热力学性质的分子水平信息。CH 4与表面路易斯酸性位点(Ca 2+,表面OH)的相互作用导致较大的诱导偶极子(〜1 D),与正常的非极性CH 4的相互作用(一旦CH 4会稳定)相比,导致相对强的吸附能形成层。插层CH如图4所示,当较少阻碍与Ca 2+阳离子的相互作用时,在较低的水合水平下也表现出大的诱导偶极子。CO 2在阳离子的配位层中(在中间层中)或在表面上置换CH 4,从而驱动CH 4的萃取。我们的模拟表明,存在一个最佳压力范围(约70–90 bar),在该压力范围内,scCO 2促进的CH 4萃取将达到最大。
更新日期:2017-11-20
中文翻译:

水合钙-蒙脱土中CH 4和CO 2吸附的分子水平研究
我们已经研究了H 2 O / CH 4 / CO 2混合物在典型的膨胀页岩成分Ca–蒙脱石上的嵌入和甲烷吸附的机理。我们在323 K和90 bar的压力下进行了从头算分子动力学模拟,以获取有关吸附能,形态以及结构和热力学性质的分子水平信息。CH 4与表面路易斯酸性位点(Ca 2+,表面OH)的相互作用导致较大的诱导偶极子(〜1 D),与正常的非极性CH 4的相互作用(一旦CH 4会稳定)相比,导致相对强的吸附能形成层。插层CH如图4所示,当较少阻碍与Ca 2+阳离子的相互作用时,在较低的水合水平下也表现出大的诱导偶极子。CO 2在阳离子的配位层中(在中间层中)或在表面上置换CH 4,从而驱动CH 4的萃取。我们的模拟表明,存在一个最佳压力范围(约70–90 bar),在该压力范围内,scCO 2促进的CH 4萃取将达到最大。











































 京公网安备 11010802027423号
京公网安备 11010802027423号