当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Total Synthesis and Absolute Configuration of Raputindole A
Organic Letters ( IF 4.9 ) Pub Date : 2017-11-17 00:00:00 , DOI: 10.1021/acs.orglett.7b03014 Mario Kock 1 , Peter G. Jones 1 , Thomas Lindel 1
Organic Letters ( IF 4.9 ) Pub Date : 2017-11-17 00:00:00 , DOI: 10.1021/acs.orglett.7b03014 Mario Kock 1 , Peter G. Jones 1 , Thomas Lindel 1
Affiliation
|
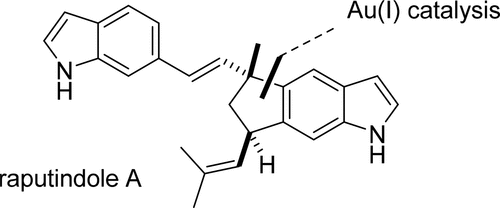
The first total synthesis of the bisindole alkaloid raputindole A from the rutaceous plant Raputia simulans is reported. The key step is a Au(I)-catalyzed cyclization that assembles the cyclopenta[f]indole tricycle from a 6-alkynylated indoline precursor. The isobutenyl side chain was installed by Suzuki–Miyaura cross-coupling, followed by a regioselective reduction employing LiDBB. Starting from 6-iodoindole, the sequence needs nine steps and provided (±)-raputindole A in 6.6% overall yield. The absolute configuration of the natural product (+)-raputindole A was determined by quantum chemical calculation of the ECD spectrum.
中文翻译:

Raputindole A的全合成和绝对构型
据报道,从芸苔植物Raputia simulans中首次合成了双吲哚生物碱RaputindoleA 。关键步骤是Au(I)催化的环化反应,该反应由6烷基化的吲哚啉前体组装成环戊[ f ]吲哚三环。异丁烯基侧链通过Suzuki-Miyaura交叉偶联安装,然后使用LiDBB进行区域选择性还原。从6-碘吲哚开始,该序列需要九个步骤,并以6.6%的总收率提供了(±)-raputindoleA。天然产物(+)-raputindole A的绝对构型是通过ECD光谱的量子化学计算确定的。
更新日期:2017-11-17
中文翻译:

Raputindole A的全合成和绝对构型
据报道,从芸苔植物Raputia simulans中首次合成了双吲哚生物碱RaputindoleA 。关键步骤是Au(I)催化的环化反应,该反应由6烷基化的吲哚啉前体组装成环戊[ f ]吲哚三环。异丁烯基侧链通过Suzuki-Miyaura交叉偶联安装,然后使用LiDBB进行区域选择性还原。从6-碘吲哚开始,该序列需要九个步骤,并以6.6%的总收率提供了(±)-raputindoleA。天然产物(+)-raputindole A的绝对构型是通过ECD光谱的量子化学计算确定的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号