当前位置:
X-MOL 学术
›
Catal. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mechanisms of metal-catalyzed cycloisomerizations of o-propargylbiaryls and o-allenylbiaryls to phenanthrenes: a DFT study
Catalysis Science & Technology ( IF 4.4 ) Pub Date : 2017-11-07 00:00:00 , DOI: 10.1039/c7cy01899a Mengistu Gemech Menkir,Shyi-Long Lee
Catalysis Science & Technology ( IF 4.4 ) Pub Date : 2017-11-07 00:00:00 , DOI: 10.1039/c7cy01899a Mengistu Gemech Menkir,Shyi-Long Lee
|
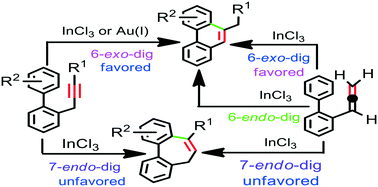
A theoretical investigation of the mechanism and regioselectivity of the InCl3- and Au(I)-catalyzed cycloisomerization of o-propargylbiphenyl as well as the InCl3-catalyzed cycloisomerization of o-allenylbiphenyl has been carried out with the aid of DFT calculations. The calculation results reveal that the Au(I)-catalyzed cycloisomerization of o-propargylbiphenyl occurs exclusively via 6-exo-dig cyclization in a Friedel–Crafts-type mechanism. The results also indicate that the 6-exo-dig cyclization is highly favored over the 7-endo-dig cyclization with InCl3 catalyst, while the mechanism via allene intermediate is less likely. In the Au(I) catalysis, the Friedel–Crafts-type intermediate is converted to the exo-double bond intermediate by a direct 1,3-H shift and then stepwise two consecutive 1,2-H shift steps leading to the final product. However, in the InCl3 catalysis intermolecular chloride-assisted H-abstraction/proto-demetalation between two Friedel–Crafts-type intermediates are more favorable than the direct 1,3-H shift. In addition, intramolecular chloride-assisted H-abstraction/proto-demetalation are more favorable than the stepwise two consecutive 1,2-H shift steps for the transformation of the exo-double bond intermediate into the final product. The feasibility of the InCl3-catalyzed cycloisomerization of o-propargylbiphenyl depends on the nature of the substituent on the phenyl ring and/or in the propargyl moiety. It is found that the cycloisomerization of electron-deficient o-propargylbiaryl as well as an unfunctionalized biaryl bearing alkyl group in the propargyl moiety may not be feasible. In contrast, InCl3-catalyzed cycloisomerization of o-propargylbiphenyl is both kinetically and thermodynamically more favorable than that of o-allenylbiphenyl. The cycloisomerization of o-allenylbiphenyl prefers 6-exo-dig over 6-endo-dig and 7-endo-dig cyclizations. Our calculation results are in good agreement with the experimental observations.
中文翻译:

金属催化的邻炔丙基联芳基和邻烯丙基联芳基化为菲的机理:DFT研究
借助于DFT计算,已经进行了InCl 3和Au(I)催化的邻炔丙基联苯的环异构化以及InCl 3催化的邻烯丙基联苯的环异构化的机理和区域选择性的理论研究。计算结果表明,Au(I)催化的邻炔丙基联苯的环异构化仅通过Friedel-Crafts型机理中的6- exo- dig环化发生。结果还表明6- exo- dig环化比7- endo高度受青睐用InCl 3催化剂进行环挖-环化,而经由烯丙基中间体的机理不太可能。在Au(I)催化中,Friedel-Crafts型中间体通过直接的1,3-H转移然后逐步进行两个连续的1,2-H转移步骤转化为exo-双键中间体,从而生成最终产物。然而,在InCl 3催化中,两个Friedel-Crafts型中间体之间的分子间氯化物辅助的H吸收/原脱金属比直接1,3-H转移更有利。此外,分子内氯化物辅助的H吸收/原脱金属比exo的转化连续两个连续的1,2-H移位步骤更有利-双键中间体进入最终产物。InCl 3催化的邻炔丙基联苯的环异构化的可行性取决于苯环和/或炔丙基部分上取代基的性质。发现缺乏电子的邻-炔丙基二芳基以及炔丙基部分中未官能化的带有烷基的联芳基的环异构化可能是不可行的。相比之下,含3的催化的环异构ö -propargylbiphenyl既是动力学和热力学比的更有利的Ó -allenylbiphenyl。邻-烯基联苯的环异构化比6--更优选6- exo- dig内切和7-内切环化。我们的计算结果与实验观察结果非常吻合。
更新日期:2017-11-17
中文翻译:

金属催化的邻炔丙基联芳基和邻烯丙基联芳基化为菲的机理:DFT研究
借助于DFT计算,已经进行了InCl 3和Au(I)催化的邻炔丙基联苯的环异构化以及InCl 3催化的邻烯丙基联苯的环异构化的机理和区域选择性的理论研究。计算结果表明,Au(I)催化的邻炔丙基联苯的环异构化仅通过Friedel-Crafts型机理中的6- exo- dig环化发生。结果还表明6- exo- dig环化比7- endo高度受青睐用InCl 3催化剂进行环挖-环化,而经由烯丙基中间体的机理不太可能。在Au(I)催化中,Friedel-Crafts型中间体通过直接的1,3-H转移然后逐步进行两个连续的1,2-H转移步骤转化为exo-双键中间体,从而生成最终产物。然而,在InCl 3催化中,两个Friedel-Crafts型中间体之间的分子间氯化物辅助的H吸收/原脱金属比直接1,3-H转移更有利。此外,分子内氯化物辅助的H吸收/原脱金属比exo的转化连续两个连续的1,2-H移位步骤更有利-双键中间体进入最终产物。InCl 3催化的邻炔丙基联苯的环异构化的可行性取决于苯环和/或炔丙基部分上取代基的性质。发现缺乏电子的邻-炔丙基二芳基以及炔丙基部分中未官能化的带有烷基的联芳基的环异构化可能是不可行的。相比之下,含3的催化的环异构ö -propargylbiphenyl既是动力学和热力学比的更有利的Ó -allenylbiphenyl。邻-烯基联苯的环异构化比6--更优选6- exo- dig内切和7-内切环化。我们的计算结果与实验观察结果非常吻合。











































 京公网安备 11010802027423号
京公网安备 11010802027423号