Molecular Cell ( IF 14.5 ) Pub Date : 2017-11-16 , DOI: 10.1016/j.molcel.2017.10.024 Jennifer L. Stamos , Alfred M. Lentzsch , Alan M. Lambowitz
|
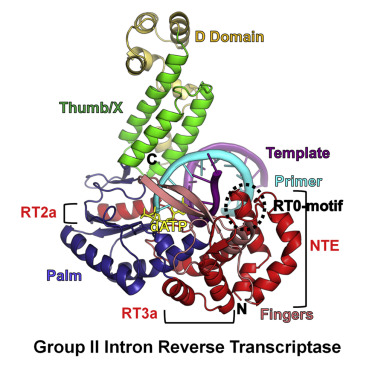
Bacterial group II intron reverse transcriptases (RTs) function in both intron mobility and RNA splicing and are evolutionary predecessors of retrotransposon, telomerase, and retroviral RTs as well as the spliceosomal protein Prp8 in eukaryotes. Here we determined a crystal structure of a full-length thermostable group II intron RT in complex with an RNA template-DNA primer duplex and incoming deoxynucleotide triphosphate (dNTP) at 3.0-Å resolution. We find that the binding of template-primer and key aspects of the RT active site are surprisingly different from retroviral RTs but remarkably similar to viral RNA-dependent RNA polymerases. The structure reveals a host of features not seen previously in RTs that may contribute to distinctive biochemical properties of group II intron RTs, and it provides a prototype for many related bacterial and eukaryotic non-LTR retroelement RTs. It also reveals how protein structural features used for reverse transcription evolved to promote the splicing of both group II and spliceosomal introns.
中文翻译:

具有模板引物的热稳定的II组内含子逆转录酶的结构及其功能和进化意义
细菌II类内含子逆转录酶(RTs)在内含子移动性和RNA剪接中均起作用,并且是真核生物中反转录转座子,端粒酶和逆转录病毒RT以及剪接体蛋白Prp8的进化前身。在这里,我们确定了全长热稳定的II组内含子RT的晶体结构,该结构与RNA模板-DNA引物双链体和传入的脱氧核苷酸三磷酸(dNTP)形成了3.0-Å的分辨率。我们发现,模板引物和RT活性位点的关键方面的结合出乎意料地不同于逆转录病毒RT,但与病毒RNA依赖性RNA聚合酶非常相似。该结构揭示了RTs以前未见的许多特征,这些特征可能有助于II组内含子RTs的独特生化特性,它提供了许多相关的细菌和真核非LTR逆转录RT的原型。它还揭示了用于逆转录的蛋白质结构特征如何进化以促进II组和剪接内含子的剪接。











































 京公网安备 11010802027423号
京公网安备 11010802027423号