当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structural Variability of 4f and 5f Thiocyanate Complexes and Dissociation of Uranium(III)–Thiocyanate Bonds with Increased Ionicity
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2017-11-16 00:00:00 , DOI: 10.1021/acs.inorgchem.7b01560 Saptarshi Biswas 1 , Shuwen Ma 2 , Stefano Nuzzo 1 , Brendan Twamley 1 , Andrew T. Russell 2 , James A. Platts 3 , František Hartl 2 , Robert J. Baker 1
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2017-11-16 00:00:00 , DOI: 10.1021/acs.inorgchem.7b01560 Saptarshi Biswas 1 , Shuwen Ma 2 , Stefano Nuzzo 1 , Brendan Twamley 1 , Andrew T. Russell 2 , James A. Platts 3 , František Hartl 2 , Robert J. Baker 1
Affiliation
|
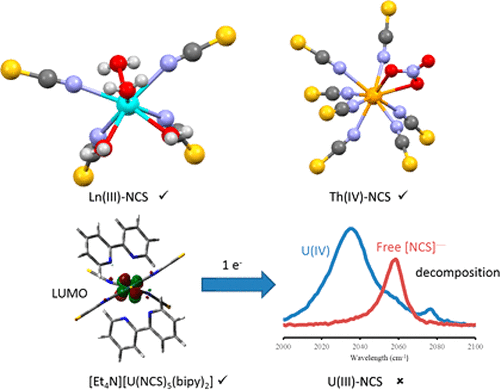
A series of complexes [Et4N][Ln(NCS)4(H2O)4] (Ln = Pr, Tb, Dy, Ho, Yb) have been structurally characterized, all showing the same structure, namely a distorted square antiprismatic coordination geometry, and the Ln–O and Ln–N bond lengths following the expected lanthanide contraction. When the counterion is Cs+, a different structural motif is observed and the eight-coordinate complex Cs5[Nd(NCS)8] isolated. The thorium compounds [Me4N]4[Th(NCS)7(NO3)] and [Me4N]4[Th(NCS)6(NO3)2] have been characterized, and high coordination numbers are also observed. Finally, attempts to synthesize a U(III) thiocyanate compound has been unsuccessful; from the reaction mixture, a heterocycle formed by condensation of five MeCN solvent molecules, possibly promoted by U(III), was isolated and structurally characterized. To rationalize the inability to isolate U(III) thiocyanate compounds, thin-layer cyclic voltammetry and IR spectroelectrochemistry have been utilized to explore the cathodic behavior of [Et4N]4[U(NCS)8] and [Et4N][U(NCS)5(bipy)2] along with a related uranyl compound [Et4N]3[UO2(NCS)5]. In all examples, the reduction triggers a rapid dissociation of [NCS]− ions and decomposition. Interestingly, the oxidation chemistry of [Et4N]3[UO2(NCS)5] in the presence of bipy gives the U(IV) compound [Et4N]4[U(NCS)8], an unusual example of a ligand-based oxidation triggering a metal-based reduction. The experimental results have been augmented by a computational investigation, concluding that the U(III)–NCS bond is more ionic than the U(IV)–NCS bond.
中文翻译:

4f和5f硫氰酸盐配合物的结构变异性和离子性增强的铀(III)-硫氰酸盐键的离解
一系列配合物[Et 4 N] [Ln(NCS)4(H 2 O)4 ](Ln = Pr,Tb,Dy,Ho,Yb)的结构特征在于,均显示相同的结构,即扭曲的正方形反棱柱形配位几何,以及预期的镧系元素收缩后的Ln–O和Ln–N键长。当抗衡离子为Cs +时,观察到不同的结构基序,并且分离出八坐标复合物Cs 5 [Nd(NCS)8 ]。compounds化合物[Me 4 N] 4 [Th(NCS)7(NO 3)]和[Me 4 N] 4 [Th(NCS)6(NO3)2 ]已经被表征,并且还观察到高配位数。最后,合成硫氰酸U(III)化合物的尝试失败。从反应混合物中分离出可能由U(III)促进的5个MeCN溶剂分子缩合形成的杂环,并对其结构进行了表征。为了合理化无法分离硫氰酸U(III)化合物,已利用薄层循环伏安法和IR光谱电化学来研究[Et 4 N] 4 [U(NCS)8 ]和[Et 4 N] [ U(NCS)5(bipy)2 ]和相关的铀酰化合物[Et 4 N] 3[UO 2(NCS)5 ]。在所有示例中,还原触发[NCS] -离子的快速解离和分解。有趣的是,在联吡啶存在下,[Et 4 N] 3 [UO 2(NCS)5 ]的氧化化学反应生成了U(IV)化合物[Et 4 N] 4 [U(NCS)8 ],这是一个不寻常的例子。基于配体的氧化引发基于金属的还原。通过计算研究增强了实验结果,认为U(III)-NCS键比U(IV)-NCS键更具离子性。
更新日期:2017-11-16
中文翻译:

4f和5f硫氰酸盐配合物的结构变异性和离子性增强的铀(III)-硫氰酸盐键的离解
一系列配合物[Et 4 N] [Ln(NCS)4(H 2 O)4 ](Ln = Pr,Tb,Dy,Ho,Yb)的结构特征在于,均显示相同的结构,即扭曲的正方形反棱柱形配位几何,以及预期的镧系元素收缩后的Ln–O和Ln–N键长。当抗衡离子为Cs +时,观察到不同的结构基序,并且分离出八坐标复合物Cs 5 [Nd(NCS)8 ]。compounds化合物[Me 4 N] 4 [Th(NCS)7(NO 3)]和[Me 4 N] 4 [Th(NCS)6(NO3)2 ]已经被表征,并且还观察到高配位数。最后,合成硫氰酸U(III)化合物的尝试失败。从反应混合物中分离出可能由U(III)促进的5个MeCN溶剂分子缩合形成的杂环,并对其结构进行了表征。为了合理化无法分离硫氰酸U(III)化合物,已利用薄层循环伏安法和IR光谱电化学来研究[Et 4 N] 4 [U(NCS)8 ]和[Et 4 N] [ U(NCS)5(bipy)2 ]和相关的铀酰化合物[Et 4 N] 3[UO 2(NCS)5 ]。在所有示例中,还原触发[NCS] -离子的快速解离和分解。有趣的是,在联吡啶存在下,[Et 4 N] 3 [UO 2(NCS)5 ]的氧化化学反应生成了U(IV)化合物[Et 4 N] 4 [U(NCS)8 ],这是一个不寻常的例子。基于配体的氧化引发基于金属的还原。通过计算研究增强了实验结果,认为U(III)-NCS键比U(IV)-NCS键更具离子性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号