当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enantioselective Excited-State Photoreactions Controlled by a Chiral Hydrogen-Bonding Iridium Sensitizer
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2017-11-16 00:00:00 , DOI: 10.1021/jacs.7b10586 Kazimer L. Skubi 1 , Jesse B. Kidd 1 , Hoimin Jung 2, 3 , Ilia A. Guzei 1 , Mu-Hyun Baik 2, 3 , Tehshik P. Yoon 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2017-11-16 00:00:00 , DOI: 10.1021/jacs.7b10586 Kazimer L. Skubi 1 , Jesse B. Kidd 1 , Hoimin Jung 2, 3 , Ilia A. Guzei 1 , Mu-Hyun Baik 2, 3 , Tehshik P. Yoon 1
Affiliation
|
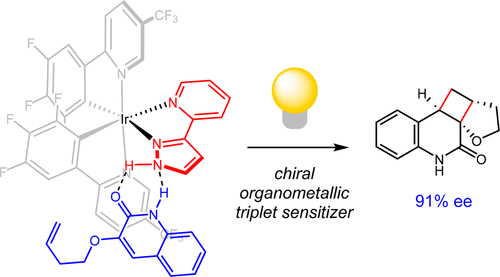
Stereochemical control of electronically excited states is a long-standing challenge in photochemical synthesis, and few catalytic systems that produce high enantioselectivities in triplet-state photoreactions are known. We report herein an exceptionally effective chiral photocatalyst that recruits prochiral quinolones using a series of hydrogen-bonding and π–π interactions. The organization of these substrates within the chiral environment of the transition-metal photosensitizer leads to efficient Dexter energy transfer and effective stereoinduction. The relative insensitivity of these organometallic chromophores toward ligand modification enables the optimization of this catalyst structure for high enantiomeric excess at catalyst loadings as much as 100-fold lower than the optimal conditions reported for analogous chiral organic photosensitizers.
中文翻译:

手性氢键合铱敏化剂控制的对映选择性激发态光反应
电子激发态的立体化学控制是光化学合成中的长期挑战,很少有人知道在三重态光反应中产生高对映选择性的催化体系。我们在这里报告了一种异常有效的手性光催化剂,它使用一系列氢键和π-π相互作用募集前手性喹诺酮。这些底物在过渡金属光敏剂的手性环境中的组织导致有效的德克斯特能量转移和有效的立体感应。这些有机金属发色团对配体修饰的相对不敏感性使得该催化剂结构的优化对于在催化剂负载量下高对映体过量的情况比报道的类似手性有机光敏剂的最佳条件低了100倍。
更新日期:2017-11-17
中文翻译:

手性氢键合铱敏化剂控制的对映选择性激发态光反应
电子激发态的立体化学控制是光化学合成中的长期挑战,很少有人知道在三重态光反应中产生高对映选择性的催化体系。我们在这里报告了一种异常有效的手性光催化剂,它使用一系列氢键和π-π相互作用募集前手性喹诺酮。这些底物在过渡金属光敏剂的手性环境中的组织导致有效的德克斯特能量转移和有效的立体感应。这些有机金属发色团对配体修饰的相对不敏感性使得该催化剂结构的优化对于在催化剂负载量下高对映体过量的情况比报道的类似手性有机光敏剂的最佳条件低了100倍。











































 京公网安备 11010802027423号
京公网安备 11010802027423号