JAMA Oncology ( IF 22.5 ) Pub Date : 2018-01-01 , DOI: 10.1001/jamaoncol.2017.3462 Andrew J. Sinnamon 1 , Madalyn G. Neuwirth 1 , Phyllis A. Gimotty 2 , Tara C. Gangadhar 3 , Ravi K. Amaravadi 3 , Lynn M. Schuchter 3 , Giorgos C. Karakousis 1
|
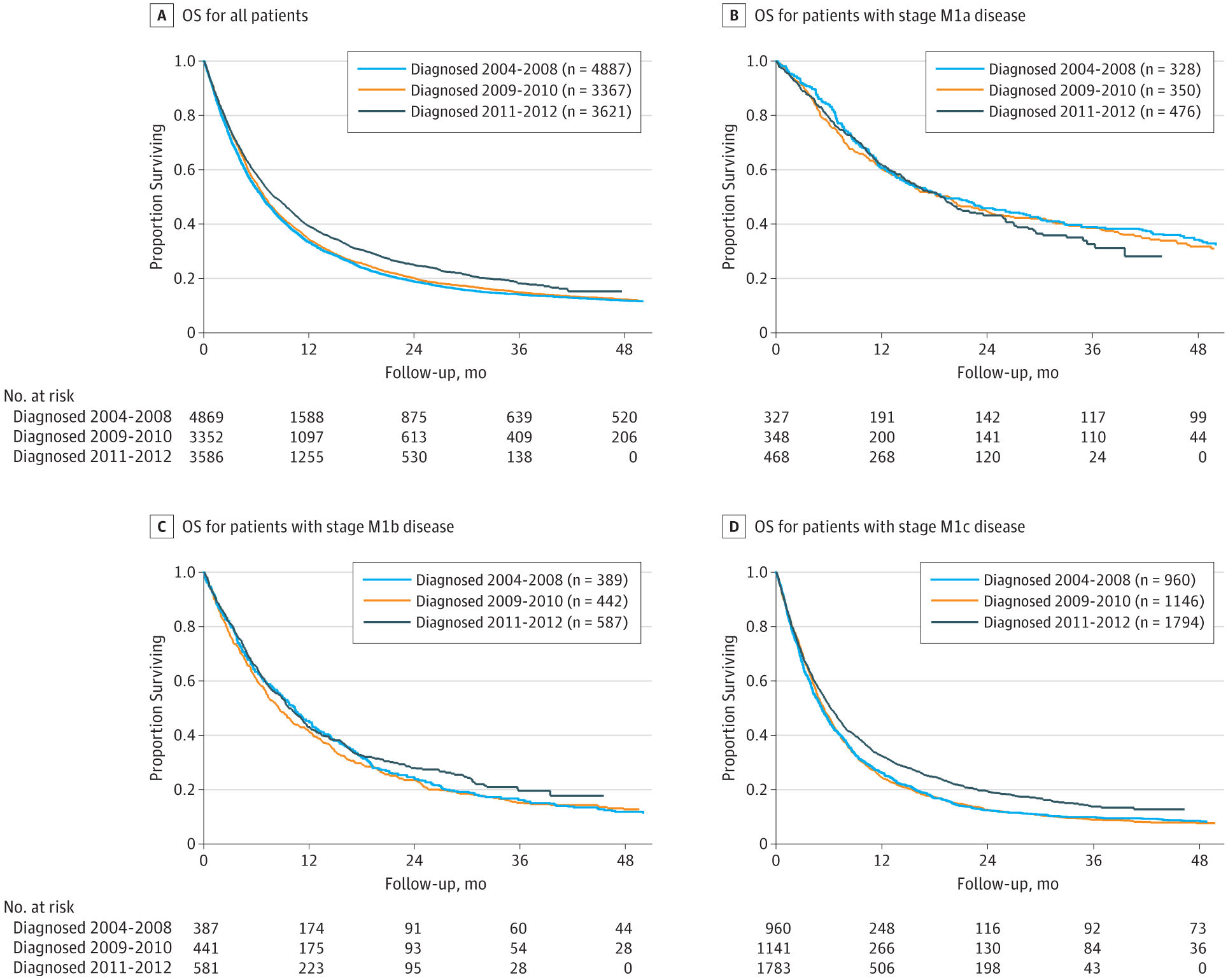
The management of advanced melanoma has witnessed dramatic changes in recent years with the rational development of novel systemic therapies. The efficacies of immune checkpoint inhibitors and targeted BRAF/MEK pathway inhibitors have been demonstrated in well-designed randomized clinical trials.1- 5 These drugs subsequently gained approval from the US Food and Drug Administration, first becoming available to patients with stage IV melanoma in the United States in 2011 with the approval of ipilimumab (March 2011) and vemurafenib (August 2011). The efficacies of these drugs have been demonstrated in the context of randomized clinical trials, but their association with patient outcomes on a population level is less well defined. We present here early findings of the initial national outcomes resulting from these therapies.
中文翻译:

IV期黑色素瘤患者一流的免疫检查点抑制和靶向治疗与生存率的关系
随着新型全身疗法的合理发展,晚期黑素瘤的治疗近年来发生了巨大变化。精心设计的随机临床试验已证明了免疫检查点抑制剂和靶向BRAF / MEK途径抑制剂的功效。1 - 5这些药物随后获得了美国食品和药物管理局的批准,并于2011年在ipilimumab(2011年3月)和vemurafenib(2011年8月)的批准下首次在美国IV期黑色素瘤患者中使用。这些药物的疗效已在随机临床试验的背景下得到证实,但在人群水平上与患者预后的关系尚不清楚。我们在这里介绍了由这些疗法产生的初步国家预后的早期发现。











































 京公网安备 11010802027423号
京公网安备 11010802027423号