JAMA Oncology ( IF 28.4 ) Pub Date : 2018-07-01 , DOI: 10.1001/jamaoncol.2017.3776 Stephanie Lheureux 1 , Marcus O. Butler 1, 2 , Blaise Clarke 3 , Mihaela C. Cristea 4 , Lainie P. Martin 5 , Katia Tonkin 6 , Gini F. Fleming 7 , Anna V. Tinker 8 , Hal W. Hirte 9 , Daliah Tsoref 1 , Helen Mackay 1 , Neesha C. Dhani 1 , Prafull Ghatage 10 , Johanne Weberpals 11 , Stephen Welch 12 , Nhu-An Pham 13 , Vinicius Motta 2 , Valentin Sotov 2 , Lisa Wang 1, 14 , Katherine Karakasis 1 , Smitha Udagani 1 , Suzanne Kamel-Reid 15 , Howard Z. Streicher 16 , Patricia Shaw 2 , Amit M. Oza 1
|
Importance Based on evidence of human papillomavirus (HPV)–induced immune evasion, immunotherapy may be an attractive strategy in cervical cancer. Ipilimumab is a fully humanized monoclonal antibody that blocks cytotoxic T-lymphocyte antigen-4 (CTLA-4), which acts to downregulate the T-cell immune response.
Objective To assess the safety and antitumor activity of ipilimumab in recurrent cervical cancer.
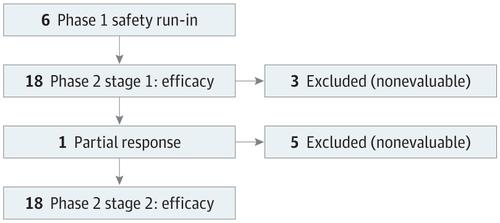
Design, Setting, and Participants A multicenter trial was designed for patients with metastatic cervical cancer (squamous cell carcinoma or adenocarcinoma) with measurable disease and progression after at least 1 line of platinum chemotherapy. A run-in safety cohort using ipilimumab, 3 mg/kg, every 21 days for 4 cycles in 6 patients was followed by a phase II cohort of ipilimumab, 10 mg/kg, every 21 days for 4 cycles and then 4 cycles of maintenance therapy every 12 weeks for patients demonstrating radiologic response or stabilization. Immune correlative studies were performed on peripheral blood before and after therapy on archival tissue and fresh tumor obtained prior to registration and 7 days after cycle 2. The study was conducted from December 3, 2012, to September 15, 2014. The data were analyzed from April 2016 to June 2016 and in July 2017.
Main Outcomes and Measures The primary end points were safety and objective response rate. Immune analyses were performed on blood and tumor tissue.
Results A total of 42 women (median age, 49 years; range, 23-78 years) were enrolled (29 [69%] squamous cell cervical cancer and 13 [31%] adenocarcinoma; 37 [93%] of 40 patients with tissue available for analysis had HPV-positive confirmation; there was no archival tissue for 2 women). Grade 3 toxic effects included diarrhea in 4 patients, 3 of whom had colitis. Of 34 patients evaluated for best response (Response Evaluation Criteria in Solid Tumors, version 1.1), 1 patient had partial response and 10 had stable disease. The median progression-free survival and overall survival were 2.5 months (95% CI, 2.1-3.2 months) and 8.5 months (95% CI, 3.6-not reached; 1 patient was still alive), respectively. Intratumoral pretreatment CD3, CD4, CD8, FoxP3, indoleamine 2,3-dioxygenase, and programmed cell death ligand 1 (PD-L1) expression was not predictive of benefit and did not significantly change with treatment. Multicolor flow cytometry on peripheral lymphocytes revealed a treatment-dependent increase of inducible T-cell costimulator, human leukocyte antigen–antigen D related, and PD-1 during initial treatment, which returned to baseline during maintenance.
Conclusions and Relevance Ipilimumab was tolerable in this population but did not show significant single-agent activity. Immune changes were induced by anti–CTLA-4 therapy but did not correlate with clinical activity. Changes in these markers may guide further treatment strategies.
中文翻译:

伊匹木单抗与转移性或复发性人乳头瘤病毒相关宫颈癌妇女的安全性和抗肿瘤活性的关系
重要性 根据人乳头瘤病毒(HPV)诱导的免疫逃逸的证据,免疫治疗可能是宫颈癌中一种有吸引力的策略。伊匹木单抗是一种完全人源化的单克隆抗体,可阻断细胞毒性T淋巴细胞抗原4(CTLA-4),从而下调T细胞免疫反应。
目的 评估依匹莫单抗在复发性宫颈癌中的安全性和抗肿瘤活性。
设计,设置和参与者 设计了一项多中心试验,针对转移性宫颈癌(鳞状细胞癌或腺癌)的患者,在至少1线铂类化疗后,其疾病和病情可测量。在6位患者中每21天使用ipilimumab 3 mg / kg,4个周期的磨合性安全队列,随后是每21天进行4个周期的ipilimumab II期队列10 mg / kg,然后4个周期的维持对于表现出放射学反应或稳定的患者,每12周进行一次治疗。对2012年12月3日至2014年9月15日进行的,在登记之前和登记后7天获得的档案组织和新鲜肿瘤进行治疗之前和之后对外周血进行的免疫相关性研究。该研究从2012年12月3日至2014年9月15日进行。 2016年4月至2016年6月以及2017年7月。
主要结果和措施 主要终点是安全性和客观响应率。对血液和肿瘤组织进行了免疫分析。
结果 共有42名女性(中位年龄49岁;范围23-78岁)入组(29名[69%]鳞状细胞癌和13名[31%]腺癌; 40名有组织的患者中有37名[93%])进行分析的人乳头瘤病毒(HPV)阳性;没有档案组织可供2名女性使用。3级毒性作用包括4例腹泻,其中3例患有结肠炎。在34位获得最佳疗效评估的患者(《实体瘤反应评估标准》,版本1.1)中,有1位患者出现部分缓解,而10位患者病情稳定。中位无进展生存期和总生存期分别为2.5个月(95%CI,2.1-3.2个月)和8.5个月(95%CI,未达到3.6; 1名患者还活着)。肿瘤内预处理CD3,CD4,CD8,FoxP3,吲哚胺2,3-二加氧酶,程序性细胞死亡配体1(PD-L1)的表达不能预测获益,并且随治疗而无明显变化。外周血淋巴细胞的多色流式细胞仪检测显示,在初始治疗期间,诱导型T细胞共刺激物,人白细胞抗原-抗原D相关和PD-1的治疗依赖性增加,在维持期间恢复至基线。
结论与相关性 伊匹木单抗在该人群中是可耐受的,但未显示出显着的单药活性。免疫改变是由抗CTLA-4治疗引起的,但与临床活动无关。这些标志物的变化可能指导进一步的治疗策略。



























 京公网安备 11010802027423号
京公网安备 11010802027423号