The Lancet ( IF 98.4 ) Pub Date : 2017-09-12 , DOI: 10.1016/s0140-6736(17)32365-6 Daniel P Petrylak , Ronald de Wit , Kim N Chi , Alexandra Drakaki , Cora N Sternberg , Hiroyuki Nishiyama , Daniel Castellano , Syed Hussain , Aude Fléchon , Aristotelis Bamias , Evan Y Yu , Michiel S van der Heijden , Nobuaki Matsubara , Boris Alekseev , Andrea Necchi , Lajos Géczi , Yen-Chuan Ou , Hasan Senol Coskun , Wen-Pin Su , Miriam Hegemann , Ivor J Percent , Jae-Lyun Lee , Marcello Tucci , Andrey Semenov , Fredrik Laestadius , Avivit Peer , Giampaolo Tortora , Sufia Safina , Xavier Garcia del Muro , Alejo Rodriguez-Vida , Irfan Cicin , Hakan Harputluoglu , Ryan C Widau , Astra M Liepa , Richard A Walgren , Oday Hamid , Annamaria H Zimmermann , Katherine M Bell-McGuinn , Thomas Powles , Suet-Lai Shirley Wong , Thean Hsiang Tan , Elizabeth Jane Hovey , Timothy Dudley Clay , Siobhan Su Wan Ng , Annemie Rutten , Jean-Pascal Machiels , Herlinde Dumez , Susanna Yee-Shan Cheng , Kim Nguyen Chi , Cristiano Ferrario , Lisa Sengeloev , Niels Viggo Jensen , Constance Thibault , Brigitte Laguerre , Fredrik Laestadius , Florence Joly , Aude Flechon , Stéphane Culine , Catherine Becht , Günter Niegisch , Michael Stöckle , Marc-Oliver Grimm , Georgios Gakis , Wolfgang Schultze-Seemann , Haralambos Kalofonos , Dimitrios Mavroudis , Christos Papandreou , Vasilis Karavasilis , Aristotelis Bamias , Janos Révész , Lajos Geczi , Eli Rosenbaum , Raya Leibowitz-Amit , Daniel Kejzman , Avivit Peer , David Sarid , Giorgio Vittorio Scagliotti , Cora N Sternberg , Giampaolo Tortora , Sergio Bracarda , Andrea Necchi , Francesco Massari , Takahiro Osawa , Naoto Miyajima , Nobuo Shinohara , Fumimasa Fukuta , Chikara Ohyama , Wataru Obara , Shinichi Yamashita , Yoshihiko Tomita , Koji Kawai , Satoshi Fukasawa , Nobuaki Matsubara , Masafumi Oyama , Junji Yonese , Masayoshi Nagata , Motohide Uemura , Kazuo Nishimura , Mutsushi Kawakita , Hiroyuki Tsunemori , Katsuyoshi Hashine , Junichi Inokuchi , Akira Yokomizo , Satoshi Nagamori , Jae-Lyun Lee , Hyo Jin Lee , Se Hoon Park , Sun Young Rha , Yu Jung Kim , Yun-Gyoo Lee , Leticia Vazquez Cortés , Claudia Lorena Urzua Flores , Reinoud J B Blaisse , Michiel S van der Heijden , Ronald de Wit , Fransiscus L G Erdkamp , Maureen J B Aarts , Joanna Wojcik-Tomaszewska , Piotr Tomczak , Bozena Sikora-Kupis , Michael Schenker , Alina Amalia Herzal , Anghel Adrian Udrea , Petr Karlov , Sufia Z Safina , Boris Alekseev , Andrey Semenov , Roman Fomkin , Enrique Grande Pulido , F Xavier García Del Muro , Juan Ignacio Delgado Mignorance , Daniel Castellano Gauna , Alejo Rodríguez-Vida , Yu-Li Su , Yen-Chuan Ou , Chien-Liang Lin , Wen-Pin Su , Chia-Chi Lin , Su-Peng Yeh , Irfan Çiçin , Hakan Harputluoglu , Mustafa Erman , Hasan Senol Coskun , Yuksel Urun , Yurii Golovko , Igor Bondarenko , Ivan Sinielnikov , Thomas Powles , Simon Crabb , Isabel Syndikus , Robert Huddart , Santhanam Sundar , Simon Chowdhury , Naveed Sarwar , Alexandra Drakaki , Thomas Flaig , Chong Xian Pan , Daniel Petrylak , James Schwarz , Ivor Percent , Jennifer Cultrera , John Hainsworth , Benjamin Herms , William Lawler , Thomas Lowe , Scott Tagawa , Jeanny Aragon-Ching , Ulka Vaishampayan
|
Background
Few treatments with a distinct mechanism of action are available for patients with platinum-refractory advanced or metastatic urothelial carcinoma. We assessed the efficacy and safety of treatment with docetaxel plus either ramucirumab—a human IgG1 VEGFR-2 antagonist—or placebo in this patient population.
Methods
We did a randomised, double-blind, phase 3 trial in patients with advanced or metastatic urothelial carcinoma who progressed during or after platinum-based chemotherapy. Patients were enrolled from 124 sites in 23 countries. Previous treatment with one immune-checkpoint inhibitor was permitted. Patients were randomised (1:1) using an interactive web response system to receive intravenous docetaxel 75 mg/m2 plus either intravenous ramucirumab 10 mg/kg or matching placebo on day 1 of repeating 21-day cycles, until disease progression or other discontinuation criteria were met. The primary endpoint was investigator-assessed progression-free survival, analysed by intention-to-treat in the first 437 randomised patients. This study is registered with ClinicalTrials.gov, number NCT02426125.
Findings
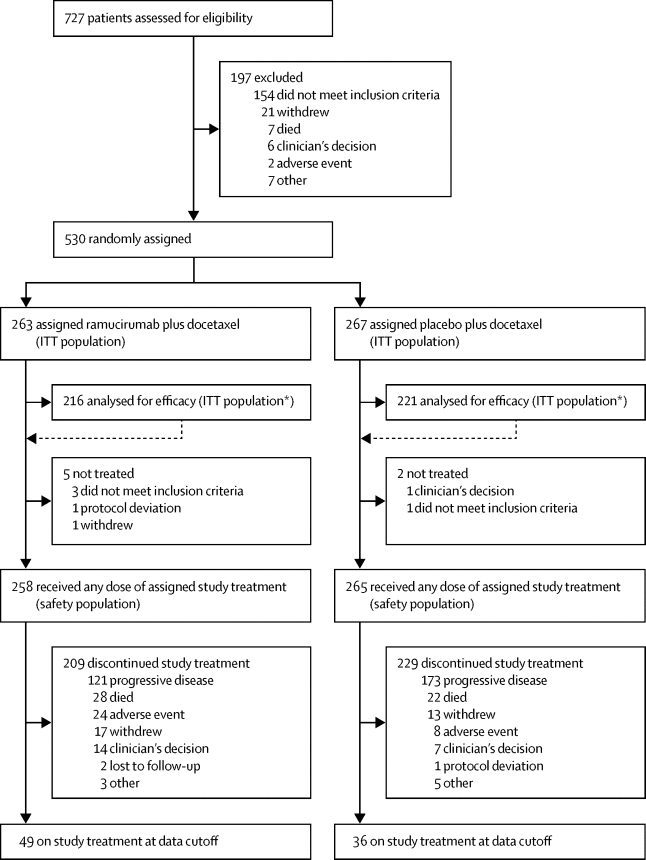
Between July, 2015, and April, 2017, 530 patients were randomly allocated either ramucirumab plus docetaxel (n=263) or placebo plus docetaxel (n=267). Progression-free survival was prolonged significantly in patients allocated ramucirumab plus docetaxel versus placebo plus docetaxel (median 4·07 months [95% CI 2·96–4·47] vs 2·76 months [2·60–2·96]; hazard ratio [HR] 0·757, 95% CI 0·607–0·943; p=0·0118). A blinded independent central analysis was consistent with these results. An objective response was achieved by 53 (24·5%, 95% CI 18·8–30·3) of 216 patients allocated ramucirumab and 31 (14·0%, 9·4–18·6) of 221 assigned placebo. The most frequently reported treatment-emergent adverse events, regardless of causality, in either treatment group (any grade) were fatigue, alopecia, diarrhoea, decreased appetite, and nausea. These events occurred predominantly at grade 1–2 severity. The frequency of grade 3 or worse adverse events was similar for patients allocated ramucirumab and placebo (156 [60%] of 258 vs 163 [62%] of 265 had an adverse event), with no unexpected toxic effects. 63 (24%) of 258 patients allocated ramucirumab and 54 (20%) of 265 assigned placebo had a serious adverse event that was judged by the investigator to be related to treatment. 38 (15%) of 258 patients allocated ramucirumab and 43 (16%) of 265 assigned placebo died on treatment or within 30 days of discontinuation, of which eight (3%) and five (2%) deaths were deemed related to treatment by the investigator. Sepsis was the most common adverse event leading to death on treatment (four [2%] vs none [0%]). One fatal event of neutropenic sepsis was reported in a patient allocated ramucirumab.
Interpretation
To the best of our knowledge, ramucirumab plus docetaxel is the first regimen in a phase 3 study to show superior progression-free survival over chemotherapy in patients with platinum-refractory advanced urothelial carcinoma. These data validate inhibition of VEGFR-2 signalling as a potential new therapeutic treatment option for patients with urothelial carcinoma.
Funding
Eli Lilly and Company.
中文翻译:

铂类治疗(RANGE)后局部晚期或转移性尿路上皮癌患者的雷米库单抗联合多西他赛与安慰剂联合多西他赛:一项随机,双盲,3期临床研究
背景
对于铂类难治性晚期或转移性尿路上皮癌患者,很少有具有独特作用机制的治疗方法。我们评估了多西他赛联合雷莫西单抗(一种人类IgG1 VEGFR-2拮抗剂)或安慰剂在该患者人群中的疗效和安全性。
方法
我们对铂类化疗期间或之后进展的晚期或转移性尿路上皮癌患者进行了一项随机,双盲,3期临床试验。来自23个国家/地区的124个站点的患者入组。允许使用一种免疫检查点抑制剂进行先前治疗。使用互动式网络反应系统将患者随机分组(1:1),在重复的21天周期的第1天接受静脉注射多西他赛75 mg / m 2加上静脉注射雷莫西单抗10 mg / kg或匹配的安慰剂,直至疾病进展或其他停药符合标准。主要终点是研究者评估的无进展生存期,在前437名随机分组的患者中通过意向性治疗进行了分析。该研究已在ClinicalTrials.gov上注册,编号NCT02426125。
发现
在2015年7月至2017年4月之间,随机分配了530例雷莫昔单抗加多西他赛(n = 263)或安慰剂加多西他赛(n = 267)。分配雷莫西单抗联合多西他赛与安慰剂联合多西他赛相比,无进展生存期显着延长(中位4·07个月[95%CI 2·96-4·47] vs2·76个月[2·60-2·96];危险比[HR] 0·757,95%CI 0·607-0·943;p = 0·0118)。盲法独立的中央分析与这些结果一致。分配了雷莫昔单抗的216例患者中有53例(24·5%,95%CI 18·8–30·3)和221例安慰剂中的31例(14·0%,9·4-18·6)实现了客观反应。不论是否因果关系,在任何一个治疗组(任何级别)中,最频繁报告的紧急治疗不良事件是疲劳,脱发,腹泻,食欲下降和恶心。这些事件主要发生在1-2级严重程度。的3级或更坏的不良事件的频率是为258患者分配ramucirumab和安慰剂(156 [60%]类似VS265例中有163例[62%]有不良反应,没有意外的毒性作用。258名接受拉莫昔单抗治疗的患者中有63名(24%)和265名接受分配的安慰剂中的54名(20%)有严重的不良事件,研究者认为这与治疗有关。接受拉莫昔单抗治疗的258名患者中有38(15%)名,而接受治疗的265名安慰剂中有43名(16%)因治疗或在停药后30天内死亡,其中8名(3%)和5名(2%)死亡被认为与治疗有关研究者。败血症是导致治疗死亡的最常见不良事件(4 [2%]比无[0%])。据报道,分配给拉莫西鲁单抗的患者发生了中性粒细胞减少性败血症的致命事件。
解释
据我们所知,雷莫昔单抗联合多西他赛是一项三期研究中的第一个方案,该方案在铂类难治性晚期尿路上皮癌患者中显示出优于化疗的无进展生存期。这些数据证实了VEGFR-2信号传导的抑制是尿路上皮癌患者潜在的新治疗选择。
资金
礼来公司。











































 京公网安备 11010802027423号
京公网安备 11010802027423号