The Lancet ( IF 98.4 ) Pub Date : 2017-09-10 , DOI: 10.1016/s0140-6736(17)32397-8 Ashley Woodcock , Jørgen Vestbo , Nawar Diar Bakerly , John New , J Martin Gibson , Sheila McCorkindale , Rupert Jones , Susan Collier , James Lay-Flurrie , Lucy Frith , Loretta Jacques , Joanne L Fletcher , Catherine Harvey , Henrik Svedsater , David Leather , David Adams-Strump , Lawrence S. Addlestone , Arash Afshar , Joann Amin , Richard Archer , Mark Austin , Ashraf Bakhat , John Behardien , Joseph M. Borg-Costanzi , Grainne Breen , Nicholas Browne , Colin Brunt , Krishnakant H. Buch , Peter Budden , Joseph Chandy , Ashish Chaudhry , Lovleen Cheema , Nagesh Chennupati , Susan Coulson , Laurence Cribbin , David Dillon , Amal El-Kafrawy , Elizabeth Elliott , Babar Farooq , Niall A. Finegan , Peter Fink , Andrew Fletcher , Sally Frier , Clare Gibbons , Lovereet Gill , Damien Herron , Brian Hope , Rachel E. Howard , Claire Hughes , Stephen Iles , Paul Jackson , Mark Jarvis , Vijaya Joshi , Naresh Kanumilli , Riaz A. Khan , Mohammed Khan , See Kwok , Nigel Lord , Chioneso Mafunga , Colin I. Malcomson , Denis K. McCarthy , Howard S. Milligan , Praful Patel , Shailesh J. Patel , Venkatachalum B. Raj , Keith A. Richardson , Ramzan Salim , Ross B. Seaton , Devang Shah , Mitesh Sharma , Harjit Singh , Nicholas Smith , Nedi N. Smyrniou , Michael Stamp , Philip Stratford-Smith , Mohammed Sultan , Ranjit S. Sumra , Jeremy Tankel , Ugo I.N. Umeadi , Colin Westwood , Julie White , Helen C. Wilkinson , Raymond G. Wilson , Simon A. Wright , Andrew T. Wright
|
Background
Evidence for management of asthma comes from closely monitored efficacy trials done in highly selected patient groups. There is a need for randomised trials that are closer to usual clinical practice.
Methods
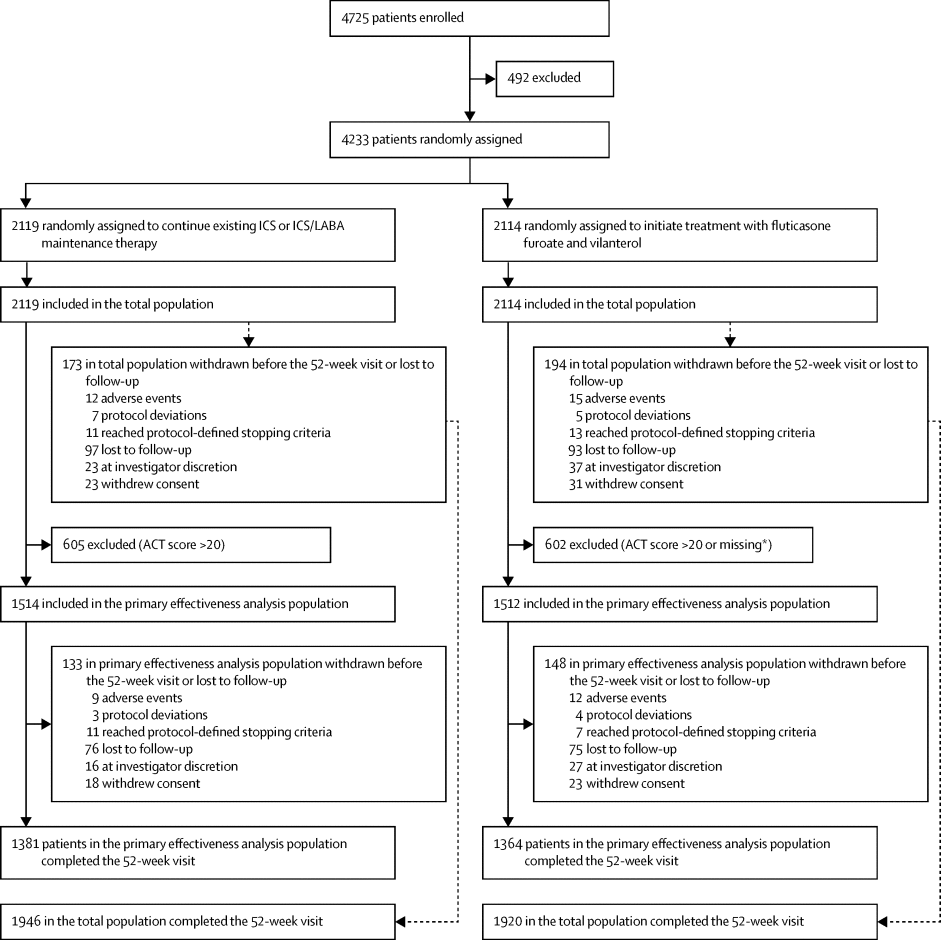
We did an open-label, randomised, controlled, two-arm effectiveness trial at 74 general practice clinics in Salford and South Manchester, UK. Patients aged 18 years or older with a general practitioner's diagnosis of symptomatic asthma and on maintenance inhaler therapy were randomly assigned to initiate treatment with a once-daily inhaled combination of either 100 μg or 200 μg fluticasone furoate with 25 μg vilanterol or optimised usual care and followed up for 12 months. The primary endpoint was the percentage of patients who achieved an asthma control test (ACT) score of 20 or greater or an increase in ACT score from baseline of 3 or greater at 24 weeks (termed responders), in patients with a baseline ACT score less than 20 (the primary effectiveness analysis population). All effectiveness analyses were done according to the intention-to-treat principle. This study is registered with ClinicalTrials.gov, number NCT01706198.
Findings
Between Nov 12, 2012, and Dec 16, 2016, 4725 patients were enrolled and 4233 randomly assigned to initiate treatment with fluticasone furoate and vilanterol (n=2114) or usual care (n=2119). 1207 patients (605 assigned to usual care, 602 to fluticasone furoate and vilanterol) had a baseline ACT score greater than or equal to 20 and were thus excluded from the primary effectiveness analysis population. At week 24, the odds of being a responder were higher for patients who initiated treatment with fluticasone furoate and vilanterol than for those on usual care (977 [71%] of 1373 in the fluticasone furoate and vilanterol group vs 784 [56%] of 1399 in the usual care group; odds ratio [OR] 2·00 [95% CI 1·70–2·34], p<0·0001). At week 24, the adjusted mean ACT score increased by 4·4 points from baseline in patients initiated with fluticasone furoate and vilanterol, compared with 2·8 points in the usual care group (difference 1·6 [95% CI 1·3–2·0], p<0·0001). This result was consistent for the duration of the study. Pneumonia was uncommon, with no differences between groups; there was no difference in other serious adverse events between the groups.
Interpretation
In patients with a general practitioner's diagnosis of symptomatic asthma and on maintenance inhaler therapy, initiation of a once-daily treatment regimen of combined fluticasone furoate and vilanterol improved asthma control without increasing the risk of serious adverse events when compared with optimised usual care.
Funding
GlaxoSmithKline.
中文翻译:

糠酸氟替卡松加维兰特罗在临床实践中对哮喘控制的有效性:一项开放标签,平行分组,随机对照试验
背景
治疗哮喘的证据来自在高度选择的患者组中进行的密切监视的功效试验。需要更接近常规临床实践的随机试验。
方法
我们在英国索尔福德和南曼彻斯特的74家普通诊所中进行了一项开放标签,随机对照,两臂有效性试验。将年龄在18岁以上且有全科医生诊断为症状性哮喘并接受维持吸入治疗的患者随机分配为开始接受每天一次吸入的100μg或200μg糠酸氟替卡松与25μg维兰特罗的组合或经过优化的常规护理的治疗随访12个月。主要终点是在24周内哮喘控制测试(ACT)得分达到20或更高或ACT得分从基线达到3或更高的患者百分比(称为响应者),基线ACT得分较低的患者所占百分比超过20(主要功效分析人群)。所有疗效分析均按照意向性治疗原则进行。该研究已向ClinicalTrials.gov,编号NCT01706198。
发现
在2012年11月12日至2016年12月16日期间,招募了4725例患者,随机分配4233例患者以糠酸氟替卡松和维兰特罗(n = 2114)或常规护理(n = 2119)进行治疗。1207名患者(605名接受常规护理,602名糠酸氟替卡松和维兰特罗)的基线ACT得分大于或等于20,因此被排除在主要疗效分析人群之外。在第24周时,开始使用糠酸氟替卡松和维兰特罗进行治疗的患者成为有反应的几率比接受常规护理的患者高(糠酸氟替卡松和维兰特罗组中1977人中的977 [71%] vs.普通护理组中的1399名患者中的784名[56%];比值比[OR] 2·00 [95%CI 1·70-2·34],p <0·0001)。在第24周时,糠酸氟替卡松和维兰特罗治疗的患者调整后的平均ACT得分较基线增加了4·4分,而常规护理组的这一平均得分为2·8分(差异1·6 [95%CI 1·3– 2·0],p <0·0001)。该结果在研究期间是一致的。肺炎不常见,各组之间无差异。两组之间的其他严重不良事件没有差异。
解释
对于全科医生诊断为症状性哮喘并维持吸入疗法的患者,与优化的常规护理相比,氟替卡松糠酸和维兰特罗的每日一次治疗方案的启动改善了哮喘的控制,而没有增加发生严重不良事件的风险。
资金
葛兰素史克。











































 京公网安备 11010802027423号
京公网安备 11010802027423号