当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Reactivity of Monolayer Protected Silver Clusters toward Excess Ligand: A Calorimetric Study
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2017-11-16 00:00:00 , DOI: 10.1021/acs.jpcc.7b07557 Ananya Baksi 1 , M. S. Bootharaju 1 , Pratap K. Chhotaray 2 , Papri Chakraborty 1 , Biswajit Mondal 1 , Shridevi Bhat 1 , Ramesh Gardas 2 , Thalappil Pradeep 1
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2017-11-16 00:00:00 , DOI: 10.1021/acs.jpcc.7b07557 Ananya Baksi 1 , M. S. Bootharaju 1 , Pratap K. Chhotaray 2 , Papri Chakraborty 1 , Biswajit Mondal 1 , Shridevi Bhat 1 , Ramesh Gardas 2 , Thalappil Pradeep 1
Affiliation
|
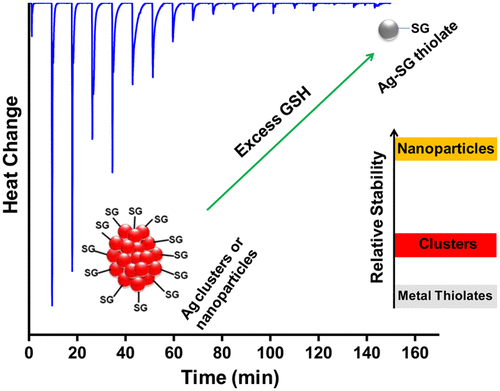
Reactivity of monolayer protected atomically precise clusters of noble metals is of significant research interest. To date very few experimental data are available on the reaction thermodynamics of such clusters. Here we report a calorimetric study of the reaction of glutathione (GSH) protected silver clusters in the presence of excess ligand, GSH, using isothermal titration calorimetry (ITC). We have studied Ag11(SG)7 and Ag32(SG)19 clusters and compared their reactivity with GSH protected silver nanoparticles (AgNPs) and silver ions. Clusters show intermediate reactivity toward excess ligand compared to nanoparticles and silver ions. Several control experiments were performed to understand the degradation mechanism of these silver clusters and nanoparticles. The effect of dissolved oxygen in the degradation process was studied in detail, and it was found that it did not have a significant role, although alternate pathways of degradation with the involvement of oxygen cannot be ruled out. Direct confirmation of the fact that functionalized metal clusters fall in-between NPs and atomic systems in their stability is obtained experimentally for the first time. Several other thermophysical parameters of these clusters were also determined, including density, speed of sound, isentropic compressibility, and coefficient of thermal expansion.
中文翻译:

单层保护的银团簇对过量配体的反应性:量热研究
单层保护的原子上精确的贵金属簇的反应性具有重要的研究兴趣。迄今为止,关于这类团簇的反应热力学的实验数据很少。在这里,我们报告了使用等温滴定量热法(ITC)对存在过量配体GSH的谷胱甘肽(GSH)保护的银簇的反应进行的量热研究。我们研究了Ag 11(SG)7和Ag 32(SG)19簇,并比较它们与GSH保护的银纳米颗粒(AgNPs)和银离子的反应性。与纳米粒子和银离子相比,团簇表现出对过量配体的中等反应性。进行了几次对照实验,以了解这些银簇和纳米颗粒的降解机理。详细研究了溶解氧在降解过程中的作用,尽管不能排除伴随氧气参与的降解途径,但溶解氧没有显着作用。首次通过实验获得了功能化金属簇的稳定性介于NP和原子系统之间的事实的直接证实。还确定了这些团簇的其他几个热物理参数,包括密度,声速,
更新日期:2017-11-16
中文翻译:

单层保护的银团簇对过量配体的反应性:量热研究
单层保护的原子上精确的贵金属簇的反应性具有重要的研究兴趣。迄今为止,关于这类团簇的反应热力学的实验数据很少。在这里,我们报告了使用等温滴定量热法(ITC)对存在过量配体GSH的谷胱甘肽(GSH)保护的银簇的反应进行的量热研究。我们研究了Ag 11(SG)7和Ag 32(SG)19簇,并比较它们与GSH保护的银纳米颗粒(AgNPs)和银离子的反应性。与纳米粒子和银离子相比,团簇表现出对过量配体的中等反应性。进行了几次对照实验,以了解这些银簇和纳米颗粒的降解机理。详细研究了溶解氧在降解过程中的作用,尽管不能排除伴随氧气参与的降解途径,但溶解氧没有显着作用。首次通过实验获得了功能化金属簇的稳定性介于NP和原子系统之间的事实的直接证实。还确定了这些团簇的其他几个热物理参数,包括密度,声速,











































 京公网安备 11010802027423号
京公网安备 11010802027423号