当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Chemoselective One-Pot Synthesis of Functionalized Amino-azaheterocycles Enabled by COware
Organic Letters ( IF 5.2 ) Pub Date : 2017-11-14 00:00:00 , DOI: 10.1021/acs.orglett.7b03214 Thomas A. Clohessy 1, 2 , Alastair Roberts 2 , Eric S. Manas 3 , Vipulkumar K. Patel 2 , Niall A. Anderson 2 , Allan J. B. Watson 1
Organic Letters ( IF 5.2 ) Pub Date : 2017-11-14 00:00:00 , DOI: 10.1021/acs.orglett.7b03214 Thomas A. Clohessy 1, 2 , Alastair Roberts 2 , Eric S. Manas 3 , Vipulkumar K. Patel 2 , Niall A. Anderson 2 , Allan J. B. Watson 1
Affiliation
|
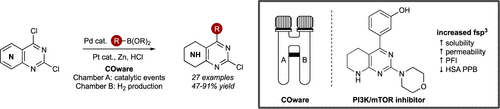
Functionalized bicyclic amino-azaheterocycles are rapidly accessed in a one-pot cross-coupling/reduction sequence enabled by the use of COware. Incompatible reagents are physically separated in a single reaction vessel to effect two chemoselective transformations—Suzuki–Miyaura cross-coupling and heteroarene reduction. The developed method allows access to novel heterocyclic templates, including semisaturated Hedgehog and dual PI3K/mTOR inhibitors, which show enhanced physicochemical properties compared to their unsaturated counterparts.
中文翻译:

COware实现的功能化氨基氮杂环杂环化合物的化学选择性一锅合成
功能化的双环氨基氮杂环化合物可以通过使用COware实现的一锅式交叉偶联/还原序列快速访问。不相容的试剂在一个反应容器中进行物理分离,以实现两个化学选择性转化-Suzuki-Miyaura交叉偶联和杂芳烃还原。所开发的方法允许访问新型杂环模板,包括半饱和刺猬和双PI3K / mTOR抑制剂,与不饱和对应物相比,它们显示出增强的理化性质。
更新日期:2017-11-14
中文翻译:

COware实现的功能化氨基氮杂环杂环化合物的化学选择性一锅合成
功能化的双环氨基氮杂环化合物可以通过使用COware实现的一锅式交叉偶联/还原序列快速访问。不相容的试剂在一个反应容器中进行物理分离,以实现两个化学选择性转化-Suzuki-Miyaura交叉偶联和杂芳烃还原。所开发的方法允许访问新型杂环模板,包括半饱和刺猬和双PI3K / mTOR抑制剂,与不饱和对应物相比,它们显示出增强的理化性质。



























 京公网安备 11010802027423号
京公网安备 11010802027423号