当前位置:
X-MOL 学术
›
RSC Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Alkyne–azide cycloaddition analogues of dehydrozingerone as potential anti-prostate cancer inhibitors via the PI3K/Akt/NF-kB pathway
RSC Medicinal Chemistry ( IF 4.1 ) Pub Date : 2017-11-02 00:00:00 , DOI: 10.1039/c7md00267j Chetan Kumar 1, 2 , Reyaz Ur Rasool 2, 3, 4, 5, 6 , Zainab Iqra 2, 5, 6, 7 , Yedukondalu Nalli 1, 2 , Prabhu Dutt 1, 2 , Naresh K. Satti 1, 2 , Neha Sharma 1, 2 , Sumit G. Gandhi 2, 6, 8, 9 , Anindya Goswami 2, 5, 6, 7 , Asif Ali 1, 2
RSC Medicinal Chemistry ( IF 4.1 ) Pub Date : 2017-11-02 00:00:00 , DOI: 10.1039/c7md00267j Chetan Kumar 1, 2 , Reyaz Ur Rasool 2, 3, 4, 5, 6 , Zainab Iqra 2, 5, 6, 7 , Yedukondalu Nalli 1, 2 , Prabhu Dutt 1, 2 , Naresh K. Satti 1, 2 , Neha Sharma 1, 2 , Sumit G. Gandhi 2, 6, 8, 9 , Anindya Goswami 2, 5, 6, 7 , Asif Ali 1, 2
Affiliation
|
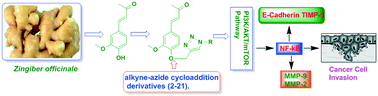
Herein, we report the isolation and synthetic modification of dehydrozingerone (DHZ, 1), a secondary metabolite present in the rhizome of Zingiber officinale. We synthesized O-propargylated dehydrozingerone, which was subsequently coupled by alkyne–azide cycloaddition (3–20) using click chemistry. The compounds (1–20) were evaluated for their in vitro cytotoxic activity in a panel of three cancer cell lines. Among all the DHZ derivatives, 3, 6, 7, 8, 9 and 15 displayed potent cytotoxic potential with an IC50 value ranging from 1.8–3.0 μM in MCF-7, PC-3 and HCT-116 cell lines. Furthermore, compound 7 has proven to be the most potent cytotoxic compound in all the three distinct cancer cell lines and also demonstrated significant anti-invasive potential in prostate cancer. The mechanistic study of compound 7 showed that it not only suppressed the AKT/mTOR signalling which regulates nuclear transcription factor-NF-kB but also augmented the expression of anti-invasive markers E-cadherin and TIMP. Compound 7 significantly decreased the expression of pro-invasive markers vimentin, MMP-2 and MMP-9, respectively. This study underscores an efficient synthetic approach employed to evaluate the structure–activity relationship of dehydrozingerone (1) in search of potential new anticancer agents.
中文翻译:

通过PI3K / Akt / NF-kB途径将脱氢姜油酮的炔-叠氮化物环加成类似物作为潜在的抗前列腺癌抑制剂
在本文中,我们报道了姜中根茎中的次生代谢产物脱氢姜油酮(DHZ,1)的分离和合成修饰。我们合成了O-炔丙基化的脱氢姜油酮,随后通过点击化学将其与炔-叠氮化物环加成(3-20)偶联。在一组三种癌细胞系中评估了化合物(1–20)的体外细胞毒性活性。在所有的DHZ衍生物,3,6,7,8,9和15显示的有效的细胞毒性潜力,其IC 50在MCF-7,PC-3和HCT-116细胞系中,该值的范围为1.8–3.0μM。此外,已证明化合物7是所有三种不同的癌细胞系中最有效的细胞毒性化合物,并且还显示出在前列腺癌中具有显着的抗侵袭潜力。化合物7的机理研究表明,它不仅抑制了调节核转录因子-NF-kB的AKT / mTOR信号传导,而且还增强了抗侵袭性标志物E-钙黏着蛋白和TIMP的表达。化合物7分别显着降低了侵袭性标志波形蛋白,MMP-2和MMP-9的表达。这项研究强调了一种有效的合成方法,用于评估脱氢姜油酮的结构与活性之间的关系(1)以寻找潜在的新型抗癌药。
更新日期:2017-11-16
中文翻译:

通过PI3K / Akt / NF-kB途径将脱氢姜油酮的炔-叠氮化物环加成类似物作为潜在的抗前列腺癌抑制剂
在本文中,我们报道了姜中根茎中的次生代谢产物脱氢姜油酮(DHZ,1)的分离和合成修饰。我们合成了O-炔丙基化的脱氢姜油酮,随后通过点击化学将其与炔-叠氮化物环加成(3-20)偶联。在一组三种癌细胞系中评估了化合物(1–20)的体外细胞毒性活性。在所有的DHZ衍生物,3,6,7,8,9和15显示的有效的细胞毒性潜力,其IC 50在MCF-7,PC-3和HCT-116细胞系中,该值的范围为1.8–3.0μM。此外,已证明化合物7是所有三种不同的癌细胞系中最有效的细胞毒性化合物,并且还显示出在前列腺癌中具有显着的抗侵袭潜力。化合物7的机理研究表明,它不仅抑制了调节核转录因子-NF-kB的AKT / mTOR信号传导,而且还增强了抗侵袭性标志物E-钙黏着蛋白和TIMP的表达。化合物7分别显着降低了侵袭性标志波形蛋白,MMP-2和MMP-9的表达。这项研究强调了一种有效的合成方法,用于评估脱氢姜油酮的结构与活性之间的关系(1)以寻找潜在的新型抗癌药。











































 京公网安备 11010802027423号
京公网安备 11010802027423号