当前位置:
X-MOL 学术
›
J. Mater. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Improved interfacial H2O supply by surface hydroxyl groups for enhanced alkaline hydrogen evolution
Journal of Materials Chemistry A ( IF 10.7 ) Pub Date : 2017-11-03 00:00:00 , DOI: 10.1039/c7ta08621k Lejuan Cai 1, 2, 3, 4 , Ziyuan Lin 1, 2, 3, 4 , Mengye Wang 1, 2, 3, 4 , Feng Pan 1, 2, 3, 4 , Jiewei Chen 1, 2, 3, 4 , Yi Wang 1, 2, 3, 4 , Xinpeng Shen 1, 2, 3, 4 , Yang Chai 1, 2, 3, 4
Journal of Materials Chemistry A ( IF 10.7 ) Pub Date : 2017-11-03 00:00:00 , DOI: 10.1039/c7ta08621k Lejuan Cai 1, 2, 3, 4 , Ziyuan Lin 1, 2, 3, 4 , Mengye Wang 1, 2, 3, 4 , Feng Pan 1, 2, 3, 4 , Jiewei Chen 1, 2, 3, 4 , Yi Wang 1, 2, 3, 4 , Xinpeng Shen 1, 2, 3, 4 , Yang Chai 1, 2, 3, 4
Affiliation
|
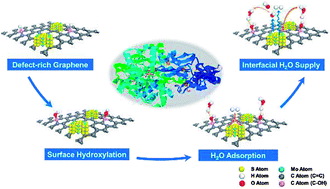
Robust hydrogen evolution reaction (HER) in alkaline solution using a cost-effective catalyst is still a critical challenge for industrial H2O electrolysis. The dramatic depletion of interfacial H2O and hindered OH− diffusion bring about a harsh interfacial environment and thereby lead to sluggish hydrogen evolution. Here we develop heterogeneous catalysts based on defect-rich three-dimensional graphene (M/3D graphene) and achieve improved HER kinetics via surface hydroxyl group modification on the graphene surface. Our theoretical studies suggest that the surface hydroxyl groups derived from the hydroxylation process effectively attract multi-overlayer H2O clusters without any thermodynamic barrier and form a reservoir to continuously supply H2O for catalytic sites. Electrochemical characterizations indicate that the HER kinetics per active electrochemical surface area have been greatly improved after crafting abundant hydroxyl groups on the 3D graphene framework, which can be attributed to sufficient proton donors and an ameliorative interfacial pH environment. Benefiting from the surface hydroxyl group modification, the MoS2/3D graphene structure facilitates the HER at a low onset potential of 60 mV in alkaline medium and exhibits excellent durability after 3000 cycles. This surface modification strategy reveals the importance of interfacial H2O supply in HER kinetics and provides general guidance on designing highly efficient catalysts in alkaline medium.
中文翻译:

表面羟基改善了界面H 2 O的供应,从而增强了碱性氢的释放
对于工业H 2 O电解而言,使用经济高效的催化剂在碱性溶液中进行强劲的氢释放反应(HER)仍然是一项关键挑战。的急剧耗尽界面ħ 2 O和受阻OH -扩散带来恶劣环境的界面,从而导致缓慢的析氢。在这里,我们开发基于富含缺陷的三维石墨烯(M / 3D石墨烯)的非均相催化剂,并通过在石墨烯表面进行表面羟基改性来提高HER动力学。我们的理论研究表明,源自羟基化过程的表面羟基可有效吸引多层H 2ø簇没有任何热力学屏障,并形成一个储存器至H连续地供给2 O代表的催化位点。电化学表征表明,在3D石墨烯骨架上精制大量羟基后,单位活性电化学表面积的HER动力学得到了极大的改善,这可以归因于足够的质子供体和改善的界面pH环境。得益于表面羟基的修饰,MoS 2 / 3D石墨烯结构在碱性介质中的60 mV的低启动电位下促进了HER,并在3000次循环后显示出出色的耐久性。这种表面改性策略揭示了界面H 2的重要性在HER动力学中提供O,并为设计在碱性介质中的高效催化剂提供一般指导。
更新日期:2017-11-16
中文翻译:

表面羟基改善了界面H 2 O的供应,从而增强了碱性氢的释放
对于工业H 2 O电解而言,使用经济高效的催化剂在碱性溶液中进行强劲的氢释放反应(HER)仍然是一项关键挑战。的急剧耗尽界面ħ 2 O和受阻OH -扩散带来恶劣环境的界面,从而导致缓慢的析氢。在这里,我们开发基于富含缺陷的三维石墨烯(M / 3D石墨烯)的非均相催化剂,并通过在石墨烯表面进行表面羟基改性来提高HER动力学。我们的理论研究表明,源自羟基化过程的表面羟基可有效吸引多层H 2ø簇没有任何热力学屏障,并形成一个储存器至H连续地供给2 O代表的催化位点。电化学表征表明,在3D石墨烯骨架上精制大量羟基后,单位活性电化学表面积的HER动力学得到了极大的改善,这可以归因于足够的质子供体和改善的界面pH环境。得益于表面羟基的修饰,MoS 2 / 3D石墨烯结构在碱性介质中的60 mV的低启动电位下促进了HER,并在3000次循环后显示出出色的耐久性。这种表面改性策略揭示了界面H 2的重要性在HER动力学中提供O,并为设计在碱性介质中的高效催化剂提供一般指导。











































 京公网安备 11010802027423号
京公网安备 11010802027423号