当前位置:
X-MOL 学术
›
Chem. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Rhodium(III)-catalyzed intramolecular annulation through C–H activation: concise synthesis of rosettacin and oxypalmatime
Chemical Communications ( IF 4.3 ) Pub Date : 2017-09-21 00:00:00 , DOI: 10.1039/c7cc06860c Liangliang Song 1, 2, 3 , Guilong Tian 1, 2, 3 , Yi He 1, 2, 3 , Erik V. Van der Eycken 1, 2, 3, 4, 5
Chemical Communications ( IF 4.3 ) Pub Date : 2017-09-21 00:00:00 , DOI: 10.1039/c7cc06860c Liangliang Song 1, 2, 3 , Guilong Tian 1, 2, 3 , Yi He 1, 2, 3 , Erik V. Van der Eycken 1, 2, 3, 4, 5
Affiliation
|
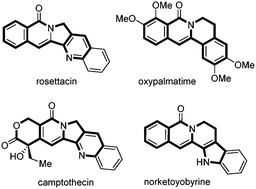
A flexible and efficient rhodium(III)-catalyzed intramolecular annulation of benzamides bearing tethered alkynes for the synthesis of indolizinones and quinolizinones is reported. This reaction shows a broad substrate scope and excellent functional-group tolerance, including different kinds of heterocyclic substrates, such as furan, thiophene, pyrrole, benzofuran, benzothiophene, indole and isonicotinamide substrates. This method also provides a practical and efficient approach for the synthesis of rosettacin and oxypalmatime.
中文翻译:

铑(III)通过C–H活化催化分子内环化:罗塞他汀和羟帕马汀的简明合成
报道了一种柔性且有效的铑(III)催化的带有链状炔烃的苯甲酰胺的分子内环化反应,用于合成吲哚嗪酮和喹啉酮。该反应显示出较宽的底物范围和优异的官能团耐受性,包括不同种类的杂环底物,例如呋喃,噻吩,吡咯,苯并呋喃,苯并噻吩,吲哚和异烟酰胺底物。该方法还提供了一种合成罗塞他汀和羟帕马汀的实用而有效的方法。
更新日期:2017-11-16
中文翻译:

铑(III)通过C–H活化催化分子内环化:罗塞他汀和羟帕马汀的简明合成
报道了一种柔性且有效的铑(III)催化的带有链状炔烃的苯甲酰胺的分子内环化反应,用于合成吲哚嗪酮和喹啉酮。该反应显示出较宽的底物范围和优异的官能团耐受性,包括不同种类的杂环底物,例如呋喃,噻吩,吡咯,苯并呋喃,苯并噻吩,吲哚和异烟酰胺底物。该方法还提供了一种合成罗塞他汀和羟帕马汀的实用而有效的方法。











































 京公网安备 11010802027423号
京公网安备 11010802027423号