当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Functional group manoeuvring for tuning stability and reactivity: synthesis of cicerfuran, moracins (D, E, M) and chromene-fused benzofuran-based natural products
Organic & Biomolecular Chemistry ( IF 3.2 ) Pub Date : 2017-11-02 00:00:00 , DOI: 10.1039/c7ob02459b Maddali L. N. Rao 1, 2, 3, 4 , Venneti N. Murty 1, 2, 3, 4 , Sachchida Nand 1, 2, 3, 4
Organic & Biomolecular Chemistry ( IF 3.2 ) Pub Date : 2017-11-02 00:00:00 , DOI: 10.1039/c7ob02459b Maddali L. N. Rao 1, 2, 3, 4 , Venneti N. Murty 1, 2, 3, 4 , Sachchida Nand 1, 2, 3, 4
Affiliation
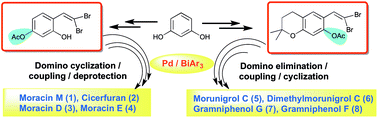
|
The protecting group manoeuvring as a strategy was applied for tuning the stability and reactivity of 4-(2,2-dibromovinyl)benzene-1,3-diol (12a) and 6-(2,2-dibromovinyl)-2,2-dimethylchroman-7-ol (22) in the domino synthesis of benzofuran-based natural products (1–8). The functional group demands and their impact on the reactivity driven by electronic effects were successfully managed by varying the protecting groups with substituted gem-dibromovinylphenols in domino couplings and triarylbismuth reagents under palladium-catalyzed conditions. This approach paved the way for the synthesis of moracin M (1) and cicerfuran (2), and the first time synthesis of moracin D (3) and moracin E (4) along with chromene-fused benzofuran-based natural products (5–8) in overall good yields.
中文翻译:

调节官能团的稳定性和反应性:合成西呋喃,草胶(D,E,M)和色烯稠合的基于苯并呋喃的天然产物
应用操纵基团作为策略来调节4-(2,2-二溴乙烯基)苯-1,3-二醇(12a)和6-(2,2-二溴乙烯基)-2,2-的稳定性和反应性基于苯并呋喃的天然产物(1-8)的多米诺合成中的二甲基苯并吡喃-7-醇(22)。在钯催化条件下,通过在多米诺偶联反应中用取代的宝石-二溴乙烯基苯酚和三芳基铋试剂改变保护基,可以成功地控制官能团的要求及其对电子效应的反应性。这种方法为合成moracin M(1)和cicerfuran(2),以及首次合成moracin D(3)铺平了道路。)和莫拉辛E(4)以及基于色烯稠合的苯并呋喃的天然产物(5-8),总体上具有良好的产量。
更新日期:2017-11-16
中文翻译:

调节官能团的稳定性和反应性:合成西呋喃,草胶(D,E,M)和色烯稠合的基于苯并呋喃的天然产物
应用操纵基团作为策略来调节4-(2,2-二溴乙烯基)苯-1,3-二醇(12a)和6-(2,2-二溴乙烯基)-2,2-的稳定性和反应性基于苯并呋喃的天然产物(1-8)的多米诺合成中的二甲基苯并吡喃-7-醇(22)。在钯催化条件下,通过在多米诺偶联反应中用取代的宝石-二溴乙烯基苯酚和三芳基铋试剂改变保护基,可以成功地控制官能团的要求及其对电子效应的反应性。这种方法为合成moracin M(1)和cicerfuran(2),以及首次合成moracin D(3)铺平了道路。)和莫拉辛E(4)以及基于色烯稠合的苯并呋喃的天然产物(5-8),总体上具有良好的产量。



























 京公网安备 11010802027423号
京公网安备 11010802027423号