当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Stereochemistry and mechanistic insights in the [2t + 2i + 2i] annulations of thioketenes and imines
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2017-10-27 00:00:00 , DOI: 10.1039/c7ob02212c Wei He 1, 2, 3, 4, 5 , Junpeng Zhuang 1, 2, 3, 4, 5 , Hongguang Du 1, 2, 3, 4, 5 , Zhanhui Yang 1, 2, 3, 4, 5 , Jiaxi Xu 1, 2, 3, 4, 5
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2017-10-27 00:00:00 , DOI: 10.1039/c7ob02212c Wei He 1, 2, 3, 4, 5 , Junpeng Zhuang 1, 2, 3, 4, 5 , Hongguang Du 1, 2, 3, 4, 5 , Zhanhui Yang 1, 2, 3, 4, 5 , Jiaxi Xu 1, 2, 3, 4, 5
Affiliation
|
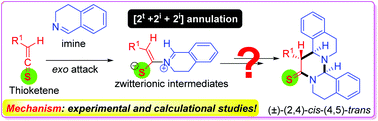
Stereochemical models and mechanistic insights are proposed for [2t + 2i + 2i] annulations of thioketenes and imines on the basis of experimental and computational investigations. In the [2t + 2i + 2i] annulations involving cyclic imines, the zwitterionic intermediates generated from monosubstituted thioketenes and the cyclic imines undergo a stepwise nucleophilic endo-addition/Si-face attack pathway with a second imines molecule, giving initially (2,4)-cis-(4,5)-cis-[2t + 2i + 2i] annuladducts, which completely epimerize into the corresponding (2,4)-cis-(4,5)-trans-annuladducts under basic reaction conditions. The annuloselectivity of thio-Staudinger cycloadditions is dependent on the substituents of both thioketenes and imines. The reactivities and annuloselectivities of Staudinger, thio-Staudinger, and sulfa-Staudinger intermediates are compared.
中文翻译:

硫酮烯和亚胺的[2 t + 2 i + 2 i ]环空中的立体化学和机理见解
在实验和计算研究的基础上,提出了[2 t + 2 i + 2 i ]硫代酮和亚胺环化的立体化学模型和机理见解。在涉及环亚胺的[2 t + 2 i + 2 i ]环中,由单取代硫代乙烯酮和环亚胺生成的两性离子中间体通过第二亚胺分子经历逐步的亲核内加成/ Si面攻击路径,最初为( 2,4)-顺式-(4,5)-顺式-[2 t + 2 i + 2 i]年环,在碱性反应条件下可完全游离为相应的(2,4)-顺式-(4,5)-反式环化产物。硫代-施陶丁格环加成的环选择性取决于硫代烯酮和亚胺的取代基。比较了施陶丁格,硫代施陶丁格和磺胺施陶丁格中间体的反应性和环选择性。
更新日期:2017-11-16
中文翻译:

硫酮烯和亚胺的[2 t + 2 i + 2 i ]环空中的立体化学和机理见解
在实验和计算研究的基础上,提出了[2 t + 2 i + 2 i ]硫代酮和亚胺环化的立体化学模型和机理见解。在涉及环亚胺的[2 t + 2 i + 2 i ]环中,由单取代硫代乙烯酮和环亚胺生成的两性离子中间体通过第二亚胺分子经历逐步的亲核内加成/ Si面攻击路径,最初为( 2,4)-顺式-(4,5)-顺式-[2 t + 2 i + 2 i]年环,在碱性反应条件下可完全游离为相应的(2,4)-顺式-(4,5)-反式环化产物。硫代-施陶丁格环加成的环选择性取决于硫代烯酮和亚胺的取代基。比较了施陶丁格,硫代施陶丁格和磺胺施陶丁格中间体的反应性和环选择性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号