当前位置:
X-MOL 学术
›
Mol. Pharmaceutics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Dual Level Statistical Investigation of Equilibrium Solubility in Simulated Fasted and Fed Intestinal Fluid
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2017-11-15 00:00:00 , DOI: 10.1021/acs.molpharmaceut.7b00869 Bayan E Ainousah 1 , Jeremy Perrier 1 , Claire Dunn 1 , Ibrahim Khadra 1 , Clive G Wilson 1 , Gavin Halbert 1
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2017-11-15 00:00:00 , DOI: 10.1021/acs.molpharmaceut.7b00869 Bayan E Ainousah 1 , Jeremy Perrier 1 , Claire Dunn 1 , Ibrahim Khadra 1 , Clive G Wilson 1 , Gavin Halbert 1
Affiliation
|
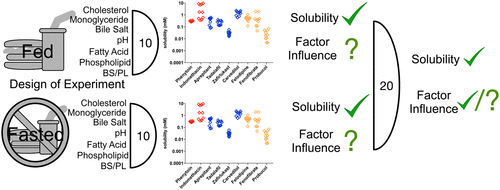
The oral route is the preferred option for drug administration but contains the inherent issue of drug absorption from the gastro-intestinal tract (GIT) in order to elicit systemic activity. A prerequisite for absorption is drug dissolution, which is dependent upon drug solubility in the variable milieu of GIT fluid, with poorly soluble drugs presenting a formulation and biopharmaceutical challenge. Multiple factors within GIT fluid influence solubility ranging from pH to the concentration and ratio of amphiphilic substances, such as phospholipid, bile salt, monoglyceride, and cholesterol. To aid in vitro investigation simulated intestinal fluids (SIF) covering the fasted and fed state have been developed. SIF media is complex and statistical design of experiment (DoE) investigations have revealed the range of solubility values possible within each state due to physiological variability along with the media factors and factor interactions which influence solubility. However, these studies require large numbers of experiments (>60) and are not feasible or sensible within a drug development setting. In the current study a smaller dual level, reduced experimental number (20) DoE providing three arms covering the fasted and fed states along with a combined analysis has been investigated. The results indicate that this small scale investigation is feasible and provides solubility ranges that encompass published data in human and simulated fasted and fed fluids. The measured fasted and fed solubility ranges are in agreement with published large scale DoE results in around half of the cases, with the differences due to changes in media composition between studies. Indicating that drug specific behaviors are being determined and that careful media factor and concentration level selection is required in order to determine a physiologically relevant solubility range. The study also correctly identifies the major single factor or factors which influence solubility but it is evident that lower significance factors (for example bile salt) are not picked up due to the lower sample number employed. A similar issue is present with factor interactions with only a limited number available for study and generally not determined to have a significant solubility impact due to the lower statistical power of the study. The study indicates that a reduced experimental number DoE is feasible, will provide solubility range results with identification of major solubility factors however statistical limitations restrict the analysis. The approach therefore represents a useful initial screening tool that can guide further in depth analysis of a drug’s behavior in gastrointestinal fluids.
中文翻译:

模拟空腹和进食肠液平衡溶解度的双级统计研究
口服途径是药物施用的优选选择,但是包含从胃肠道(GIT)吸收药物的固有问题,以引起全身活性。吸收的先决条件是药物溶解,这取决于药物在GIT液可变环境中的溶解度,而难溶药物则对制剂和生物制药构成挑战。GIT流体中的多种因素影响溶解度,范围从pH值到两亲物质(例如磷脂,胆汁盐,甘油一酸酯和胆固醇)的浓度和比率。为了帮助进行体外研究,已经开发出涵盖禁食和进食状态的模拟肠液(SIF)。SIF介质很复杂,实验的统计设计(DoE)研究表明,由于生理变异以及影响溶解度的介质因素和因素相互作用,每种状态下可能的溶解度值范围。但是,这些研究需要大量实验(> 60),并且在药物开发环境中不可行或不合理。在当前的研究中,已经研究了一种较小的双水平,减少的实验数量(20)的DoE,该DoE提供了三个涵盖空腹和进食状态的手臂,并进行了组合分析。结果表明,这种小规模的研究是可行的,并提供了涵盖人类和模拟禁食和进食液体中已公开数据的溶解度范围。在大约一半的病例中,测得的禁食和进食溶解度范围与已发表的大规模DOE结果一致,差异是由于研究之间培养基组成的变化所致。表明正在确定药物的特定行为,并且需要仔细选择培养基因子和浓度水平,才能确定生理上相关的溶解度范围。该研究还正确地确定了影响溶解度的一个或多个主要单一因素,但很明显,由于使用的样本数较少,因此未提取出较低显着性的因素(例如,胆汁盐)。因子相互作用也存在类似的问题,只有有限数量的因子可供研究,并且由于该研究的统计能力较低,因此通常不被确定具有显着的溶解性影响。研究表明,减少实验数量的DoE是可行的,它将提供溶解度范围的结果,并识别主要的溶解度因子,但是统计限制会限制分析。因此,该方法代表了一种有用的初始筛选工具,可以进一步指导对药物在胃肠液中行为的深度分析。
更新日期:2017-11-16
中文翻译:

模拟空腹和进食肠液平衡溶解度的双级统计研究
口服途径是药物施用的优选选择,但是包含从胃肠道(GIT)吸收药物的固有问题,以引起全身活性。吸收的先决条件是药物溶解,这取决于药物在GIT液可变环境中的溶解度,而难溶药物则对制剂和生物制药构成挑战。GIT流体中的多种因素影响溶解度,范围从pH值到两亲物质(例如磷脂,胆汁盐,甘油一酸酯和胆固醇)的浓度和比率。为了帮助进行体外研究,已经开发出涵盖禁食和进食状态的模拟肠液(SIF)。SIF介质很复杂,实验的统计设计(DoE)研究表明,由于生理变异以及影响溶解度的介质因素和因素相互作用,每种状态下可能的溶解度值范围。但是,这些研究需要大量实验(> 60),并且在药物开发环境中不可行或不合理。在当前的研究中,已经研究了一种较小的双水平,减少的实验数量(20)的DoE,该DoE提供了三个涵盖空腹和进食状态的手臂,并进行了组合分析。结果表明,这种小规模的研究是可行的,并提供了涵盖人类和模拟禁食和进食液体中已公开数据的溶解度范围。在大约一半的病例中,测得的禁食和进食溶解度范围与已发表的大规模DOE结果一致,差异是由于研究之间培养基组成的变化所致。表明正在确定药物的特定行为,并且需要仔细选择培养基因子和浓度水平,才能确定生理上相关的溶解度范围。该研究还正确地确定了影响溶解度的一个或多个主要单一因素,但很明显,由于使用的样本数较少,因此未提取出较低显着性的因素(例如,胆汁盐)。因子相互作用也存在类似的问题,只有有限数量的因子可供研究,并且由于该研究的统计能力较低,因此通常不被确定具有显着的溶解性影响。研究表明,减少实验数量的DoE是可行的,它将提供溶解度范围的结果,并识别主要的溶解度因子,但是统计限制会限制分析。因此,该方法代表了一种有用的初始筛选工具,可以进一步指导对药物在胃肠液中行为的深度分析。











































 京公网安备 11010802027423号
京公网安备 11010802027423号