当前位置:
X-MOL 学术
›
Cryst. Growth Des.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Variations of Structures and Phenoxazinone Synthase-like Activity of the Complexes Based on (CuII)2MnII Node and Dicyanamide Spacer
Crystal Growth & Design ( IF 3.2 ) Pub Date : 2017-11-15 00:00:00 , DOI: 10.1021/acs.cgd.7b01407 Prithwish Mahapatra 1 , Michael G. B. Drew 2 , Ashutosh Ghosh 1
Crystal Growth & Design ( IF 3.2 ) Pub Date : 2017-11-15 00:00:00 , DOI: 10.1021/acs.cgd.7b01407 Prithwish Mahapatra 1 , Michael G. B. Drew 2 , Ashutosh Ghosh 1
Affiliation
|
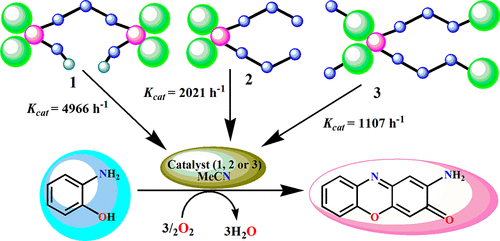
Three new heterometallic Cu(II)–Mn(II) complexes, [{(CuL)2Mn}2(μ1,5-N(CN)2)(CH3CN)2](ClO4)3 (1), [(CuL)2Mn(N(CN)2)2]·(H2O) (2), and [(CuL)2Mn(μ1,5-N(CN)2)2]n (3), have been synthesized using a Cu(II)-metalloligand of an asymmetrically dicondensed Schiff base ligand (where H2L = N-α-methylsalicylidene-N′-salicylidene-1,3-propanediamine). Complex 1 was formed when the ratio of [CuL]/Mn(ClO4)2/NaN(CN)2 was 2:1:1, whereas complexes 2 and 3 were obtained with a 2:1:2 ratio of the same reactants on varying the reaction conditions. Single-crystal structural analyses reveal that complex 1 possesses a hexanuclear structure in which two (CuII)2MnII units are connected by one μ1,5-N(CN)2– bridge, 2 is a discrete trinuclear species with two terminally coordinated N(CN)2– ions to the Mn(II), whereas complex 3 is a polymeric form of 2 with μ1,5-N(CN)2– bridges between Cu(II) and Mn(II) centers. The thermal variations of dc magnetic susceptibilities suggest that all three complexes (1–3) are antiferromagnetically coupled with comparable exchange coupling constants (−25.4, −22.8, and −22.0 cm–1, respectively) which are expected from the Cu–O–Mn angles. All the complexes show biomemitic phenoxazinone synthase-like activity for the aerial oxidation of o-aminophenol to amino phenoxazinone. The turnover numbers (kcat) for the process are 4966, 2021, and 1107 h–1 for complexes 1–3 respectively. The mass spectral evidence on intermediates suggests that the cooperative activity of the two different metal ions, i.e., coordination of substrate to Mn(II) and shuttling of oxidation state of Cu between I and II, is possibly operative in the oxidation process. The highest catalytic activity of 1 is attributed to the presence of one coordinating solvent molecule to Mn(II).
中文翻译:

(Cu II)2 Mn II节点和双氰胺间隔基的配合物的结构和苯恶嗪酮合酶活性的变化
三种新的杂金属Cu(II)-Mn(II)络合物[[{(CuL)2 Mn} 2(μ1,5 -N(CN)2)(CH 3 CN)2 ](ClO 4)3(1) ,[(CuL)2 Mn(N(CN)2)2 ]·(H 2 O)(2)和[(CuL)2 Mn(μ1,5 -N(CN)2)2 ] n(3),是使用不对称缩合的席夫碱配体(其中H 2 L = N-α-甲基水杨基-N′-水杨基-1,3-丙二胺)。复杂1形成时的比[CUL] /锰(CLO 4)2 /的NaN(CN)2为2:1:1,而复合物2和3:1:2的比例相同的反应物,用一个2获得改变反应条件 单晶结构分析揭示了复杂的1具有其中两个(Cu等六核结构II)2锰II单元由一个μ连接1,5- -N(CN)2 -桥,2是离散的三核物种具有两个末端协调N(CN)2 -离子中的Mn(II),而复杂3是的聚合形式2与μ 1,5 -N(CN)2 -铜之间的桥(II)和Mn(II)中心。的热变化的直流磁化率表明,所有三种复合物(1 - 3)的反铁磁耦合具有可比交换耦合常数(-25.4,-22.8,-22.0和厘米-1分别),其与预期的Cu-O-锰角。所有络合物显示phenoxazinone合酶像的空中氧化活性biomemitic Ø-氨基苯酚合成氨基苯恶嗪酮。的转换数(ķ猫的过程)是4966,2021,和1107ħ -1为络合物1 - 3分别。中间体的质谱证据表明,两种不同金属离子的协同活性,即底物与Mn(II)的配位以及I和II之间Cu的氧化态的穿梭,可能在氧化过程中起作用。的最高的催化活性1都归因于一个配位溶剂分子与Mn(II)的存在。
更新日期:2017-11-16
中文翻译:

(Cu II)2 Mn II节点和双氰胺间隔基的配合物的结构和苯恶嗪酮合酶活性的变化
三种新的杂金属Cu(II)-Mn(II)络合物[[{(CuL)2 Mn} 2(μ1,5 -N(CN)2)(CH 3 CN)2 ](ClO 4)3(1) ,[(CuL)2 Mn(N(CN)2)2 ]·(H 2 O)(2)和[(CuL)2 Mn(μ1,5 -N(CN)2)2 ] n(3),是使用不对称缩合的席夫碱配体(其中H 2 L = N-α-甲基水杨基-N′-水杨基-1,3-丙二胺)。复杂1形成时的比[CUL] /锰(CLO 4)2 /的NaN(CN)2为2:1:1,而复合物2和3:1:2的比例相同的反应物,用一个2获得改变反应条件 单晶结构分析揭示了复杂的1具有其中两个(Cu等六核结构II)2锰II单元由一个μ连接1,5- -N(CN)2 -桥,2是离散的三核物种具有两个末端协调N(CN)2 -离子中的Mn(II),而复杂3是的聚合形式2与μ 1,5 -N(CN)2 -铜之间的桥(II)和Mn(II)中心。的热变化的直流磁化率表明,所有三种复合物(1 - 3)的反铁磁耦合具有可比交换耦合常数(-25.4,-22.8,-22.0和厘米-1分别),其与预期的Cu-O-锰角。所有络合物显示phenoxazinone合酶像的空中氧化活性biomemitic Ø-氨基苯酚合成氨基苯恶嗪酮。的转换数(ķ猫的过程)是4966,2021,和1107ħ -1为络合物1 - 3分别。中间体的质谱证据表明,两种不同金属离子的协同活性,即底物与Mn(II)的配位以及I和II之间Cu的氧化态的穿梭,可能在氧化过程中起作用。的最高的催化活性1都归因于一个配位溶剂分子与Mn(II)的存在。











































 京公网安备 11010802027423号
京公网安备 11010802027423号