当前位置:
X-MOL 学术
›
Lancet Neurol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
24-month intervention with a specific multinutrient in people with prodromal Alzheimer's disease (LipiDiDiet): a randomised, double-blind, controlled trial
The Lancet Neurology ( IF 46.5 ) Pub Date : 2017-12-01 , DOI: 10.1016/s1474-4422(17)30332-0 Hilkka Soininen , Alina Solomon , Pieter Jelle Visser , Suzanne B Hendrix , Kaj Blennow , Miia Kivipelto , Tobias Hartmann , Ilona Hallikainen , Merja Hallikainen , Seppo Helisalmi , Tarja Lappalainen , Yawu Liu , Teemu Paajanen , Lars-Olof Wahlund , Yvonne Freund-Levi , Niels Andreasen , Göran Hagman , Stina Lindblom , Klaus Fassbender , Matthias Riemenschneider , Marcus OW Grimm , Aline Klees-Rollmann , Maxine Luley , Epameinondas Lyros , Robert Schomburg , Jennifer Kennel , Daniela Ramelli , Lutz Frölich , Lucrezia Hausner , Christoph Laske , Thomas Leyhe , Christian Mychajliw , Niklas Koehler , Stephan Schiekofer , Hans Klünemann , Johannes Schröder , Dieter Lütjohann , Philip Scheltens , Ineke van Rossum , Nienke Scheltens , Daniela Bertens , Mara ten Kate , Frederik Barkhof , Johanna ML Henselmans , Gerwin Roks , Anneke MJ van Hees , Noel Ellison
The Lancet Neurology ( IF 46.5 ) Pub Date : 2017-12-01 , DOI: 10.1016/s1474-4422(17)30332-0 Hilkka Soininen , Alina Solomon , Pieter Jelle Visser , Suzanne B Hendrix , Kaj Blennow , Miia Kivipelto , Tobias Hartmann , Ilona Hallikainen , Merja Hallikainen , Seppo Helisalmi , Tarja Lappalainen , Yawu Liu , Teemu Paajanen , Lars-Olof Wahlund , Yvonne Freund-Levi , Niels Andreasen , Göran Hagman , Stina Lindblom , Klaus Fassbender , Matthias Riemenschneider , Marcus OW Grimm , Aline Klees-Rollmann , Maxine Luley , Epameinondas Lyros , Robert Schomburg , Jennifer Kennel , Daniela Ramelli , Lutz Frölich , Lucrezia Hausner , Christoph Laske , Thomas Leyhe , Christian Mychajliw , Niklas Koehler , Stephan Schiekofer , Hans Klünemann , Johannes Schröder , Dieter Lütjohann , Philip Scheltens , Ineke van Rossum , Nienke Scheltens , Daniela Bertens , Mara ten Kate , Frederik Barkhof , Johanna ML Henselmans , Gerwin Roks , Anneke MJ van Hees , Noel Ellison
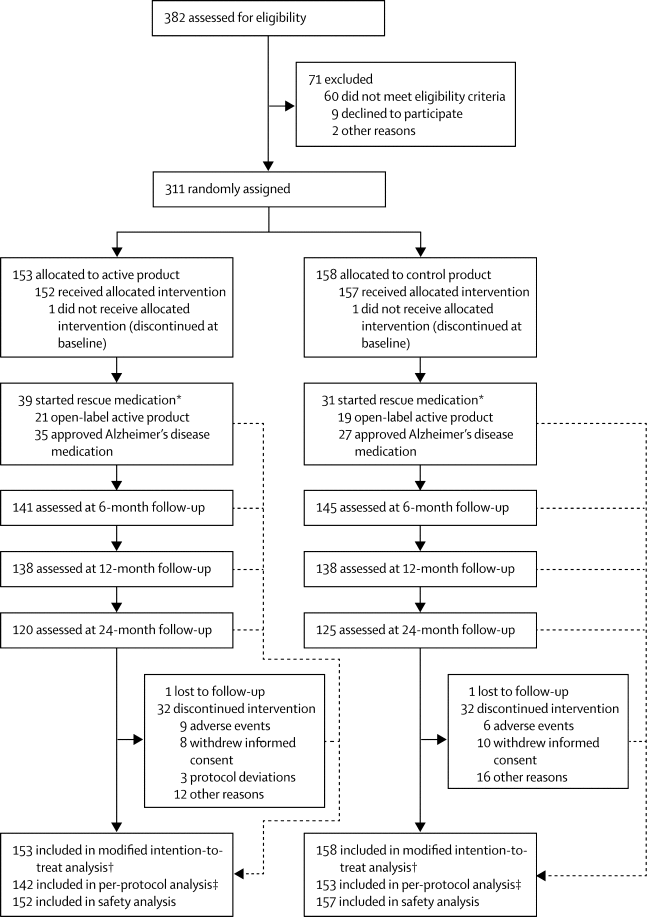
|
Summary Background Nutrition is an important modifiable risk factor in Alzheimer's disease. Previous trials of the multinutrient Fortasyn Connect showed benefits in mild Alzheimer's disease dementia. LipiDiDiet investigated the effects of Fortasyn Connect on cognition and related measures in prodromal Alzheimer's disease. Here, we report the 24-month results of the trial. Methods LipiDiDiet was a 24-month randomised, controlled, double-blind, parallel-group, multicentre trial (11 sites in Finland, Germany, the Netherlands, and Sweden), with optional 12-month double-blind extensions. The trial enrolled individuals with prodromal Alzheimer's disease, defined according to the International Working Group (IWG)-1 criteria. Participants were randomly assigned (1:1) to active product (125 mL once-a-day drink containing Fortasyn Connect) or control product. Randomisation was computer-generated centrally in blocks of four, stratified by site. All study personnel and participants were masked to treatment assignment. The primary endpoint was change in a neuropsychological test battery (NTB) score. Analysis was by modified intention to treat. Safety analyses included all participants who consumed at least one study product dose. This trial is registered with the Dutch Trial Register, number NTR1705. Findings Between April 20, 2009, and July 3, 2013, 311 of 382 participants screened were randomly assigned to the active group (n=153) or control group (n=158). Mean change in NTB primary endpoint was −0·028 (SD 0·453) in the active group and −0·108 (0·528) in the control group; estimated mean treatment difference was 0·098 (95% CI −0·041 to 0·237; p=0·166). The decline in the control group was less than the prestudy estimate of −0·4 during 24 months. 66 (21%) participants dropped out of the study. Serious adverse events occurred in 34 (22%) participants in the active group and 30 (19%) in control group (p=0·487), none of which were regarded as related to the study intervention. Interpretation The intervention had no significant effect on the NTB primary endpoint over 2 years in prodromal Alzheimer's disease. However, cognitive decline in this population was much lower than expected, rendering the primary endpoint inadequately powered. Group differences on secondary endpoints of disease progression measuring cognition and function and hippocampal atrophy were observed. Further study of nutritional approaches with larger sample sizes, longer duration, or a primary endpoint more sensitive in this pre-dementia population, is needed. Funding European Commission 7th Framework Programme.
中文翻译:

前驱阿尔茨海默病 (LipiDiDiet) 患者使用特定多营养素干预 24 个月:一项随机、双盲、对照试验
总结 背景 营养是阿尔茨海默病的一个重要的可改变风险因素。先前对多营养素 Fortasyn Connect 的试验显示出对轻度阿尔茨海默病痴呆的益处。LipiDiDiet 研究了 Fortasyn Connect 对前驱阿尔茨海默病认知和相关措施的影响。在这里,我们报告了 24 个月的试验结果。方法 LipiDiDiet 是一项为期 24 个月的随机、对照、双盲、平行组、多中心试验(芬兰、德国、荷兰和瑞典的 11 个地点),可选择进行为期 12 个月的双盲扩展。该试验招募了根据国际工作组 (IWG)-1 标准定义的前驱阿尔茨海默病患者。参与者被随机分配(1:1) 到活性产品(125 mL 每天一次的含有 Fortasyn Connect 的饮料)或对照产品。随机化是由计算机集中生成的,以四个为一组,按站点分层。所有研究人员和参与者都对治疗分配不知情。主要终点是神经心理学测试组 (NTB) 分数的变化。分析是通过改良的意向治疗。安全性分析包括所有服用至少一剂研究产品的参与者。该试验在荷兰试验登记处注册,编号 NTR1705。结果 在 2009 年 4 月 20 日和 2013 年 7 月 3 日之间,筛选的 382 名参与者中的 311 名被随机分配到活动组(n=153)或对照组(n=158)。活性组中 NTB 主要终点的平均变化为 -0·028 (SD 0·453),对照组为 -0·108 (0·528);估计的平均治疗差异为 0·098(95% CI -0·041 至 0·237;p=0·166)。在 24 个月内,对照组的下降幅度小于研究前估计的 -0·4。66 (21%) 名参与者退出研究。活性组的 34 名 (22%) 参与者和对照组的 30 名 (19%) 参与者发生严重不良事件 (p=0·487),均未认为与研究干预有关。解释 干预对前驱阿尔茨海默病 2 年内的 NTB 主要终点没有显着影响。然而,该人群的认知下降远低于预期,导致主要终点的效力不足。观察到测量认知和功能以及海马萎缩的疾病进展次要终点的组间差异。需要进一步研究具有更大样本量、更长持续时间或在该痴呆前期人群中更敏感的主要终点的营养方法。资助欧盟委员会第七框架计划。
更新日期:2017-12-01
中文翻译:

前驱阿尔茨海默病 (LipiDiDiet) 患者使用特定多营养素干预 24 个月:一项随机、双盲、对照试验
总结 背景 营养是阿尔茨海默病的一个重要的可改变风险因素。先前对多营养素 Fortasyn Connect 的试验显示出对轻度阿尔茨海默病痴呆的益处。LipiDiDiet 研究了 Fortasyn Connect 对前驱阿尔茨海默病认知和相关措施的影响。在这里,我们报告了 24 个月的试验结果。方法 LipiDiDiet 是一项为期 24 个月的随机、对照、双盲、平行组、多中心试验(芬兰、德国、荷兰和瑞典的 11 个地点),可选择进行为期 12 个月的双盲扩展。该试验招募了根据国际工作组 (IWG)-1 标准定义的前驱阿尔茨海默病患者。参与者被随机分配(1:1) 到活性产品(125 mL 每天一次的含有 Fortasyn Connect 的饮料)或对照产品。随机化是由计算机集中生成的,以四个为一组,按站点分层。所有研究人员和参与者都对治疗分配不知情。主要终点是神经心理学测试组 (NTB) 分数的变化。分析是通过改良的意向治疗。安全性分析包括所有服用至少一剂研究产品的参与者。该试验在荷兰试验登记处注册,编号 NTR1705。结果 在 2009 年 4 月 20 日和 2013 年 7 月 3 日之间,筛选的 382 名参与者中的 311 名被随机分配到活动组(n=153)或对照组(n=158)。活性组中 NTB 主要终点的平均变化为 -0·028 (SD 0·453),对照组为 -0·108 (0·528);估计的平均治疗差异为 0·098(95% CI -0·041 至 0·237;p=0·166)。在 24 个月内,对照组的下降幅度小于研究前估计的 -0·4。66 (21%) 名参与者退出研究。活性组的 34 名 (22%) 参与者和对照组的 30 名 (19%) 参与者发生严重不良事件 (p=0·487),均未认为与研究干预有关。解释 干预对前驱阿尔茨海默病 2 年内的 NTB 主要终点没有显着影响。然而,该人群的认知下降远低于预期,导致主要终点的效力不足。观察到测量认知和功能以及海马萎缩的疾病进展次要终点的组间差异。需要进一步研究具有更大样本量、更长持续时间或在该痴呆前期人群中更敏感的主要终点的营养方法。资助欧盟委员会第七框架计划。











































 京公网安备 11010802027423号
京公网安备 11010802027423号