当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Chemical Sensitivity of Valence-to-Core X-ray Emission Spectroscopy Due to the Ligand and the Oxidation State: A Computational Study on Cu-SSZ-13 with Multiple H2O and NH3 Adsorption
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2017-11-13 00:00:00 , DOI: 10.1021/acs.jpcc.7b04309 Renqin Zhang , Hui Li 1 , Jean-Sabin McEwen 2
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2017-11-13 00:00:00 , DOI: 10.1021/acs.jpcc.7b04309 Renqin Zhang , Hui Li 1 , Jean-Sabin McEwen 2
Affiliation
|
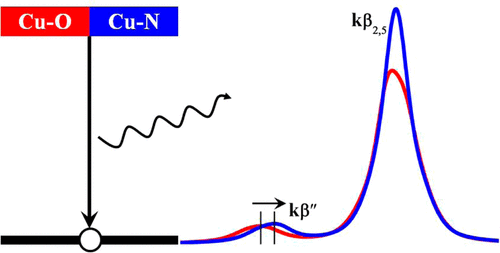
Valence-to-core X-ray emission spectroscopy (vtc-XES) is a powerful experimental tool that can overcome the sensitivity limitations of X-ray absorption near edge structure (XANES) measurements with regard to ligand identification. To further elucidate the sensitivity of this experimental technique, the corresponding emission spectrum of a Cu cation when exchanged within a chabazite structure (Cu-SSZ-13) was calculated from first principles. By comparing vtc-XES spectra of H2O and NH3 adsorbed on Cu+ (or Cu2+) cations, we find a blue shift for the kβ″ lines from a Cu–O to a Cu–N ligation. In addition, the adsorption of NH3 results in a stronger kβ2,5 line intensity than the corresponding one for H2O. Therefore, one can discriminate the adsorption of H2O or NH3 by the different vtc-XES emission lines of Cu–O and Cu–N. By scanning the vtc-XES of multiple H2O and NH3 adsorbed Cu-SSZ-13 structures, we find a shift in the energy of the kβ″ line between H2O and NH3 adsorbed conformations, which increases by increasing the population of Cu–ligand bonds for both the Cu+ and Cu2+ cations. By analyzing the partial density of states (PDOS) for these structures, the kβ″ emission line results from a N 2s to Cu 1s transition, while the kβ2,5 emission line is generated from the transition going from a mixed N 2p and Cu 3d and 4p state to a Cu 1s core hole, where the Cu 4p state plays a key role in this transition. Further PDOS analysis shows the chemical sensitivity of vtc-XES, since the ligand environment is intrinsically determined by the different potential binding energies of ligand 2s states. Finally, we compare our computed XES results to the measured XES of several reference compounds, which seem to suggest a different assignment than what was suggested in the literature. As such, the vtc-XES spectrum is a complementary tool to XANES and is well-suited for uncovering the state of the active site and the nearest neighbor environment of Cu ions in Cu-SSZ-13 during the selective catalytic reduction of NO or, more generally, of metal ions in a working catalyst.
中文翻译:

配体和氧化态对化合价X射线电子发射光谱的化学敏感性:多次H 2 O和NH 3吸附的Cu-SSZ-13的计算研究
价核X射线发射光谱(vtc-XES)是一种功能强大的实验工具,可以克服X射线吸收近边缘结构(XANES)测量对配体识别的灵敏度限制。为了进一步阐明该实验技术的敏感性,根据第一原理计算了在菱沸石结构(Cu-SSZ-13)中交换时相应的Cu阳离子发射光谱。通过比较吸附在Cu +(或Cu 2+)阳离子上的H 2 O和NH 3的vtc-XES光谱,我们发现kβ''线从Cu–O到Cu–N的连接发生了蓝移。此外,NH吸附3所导致更强的Kβ 2,5-线的强度要比对应的H 2 O的强度大。因此,可以区分Cu–O和Cu–N的不同vtc-XES发射线对H 2 O或NH 3的吸附。通过扫描多个H 2 O和NH 3吸附的Cu-SSZ-13结构的vtc-XES ,我们发现H 2 O和NH 3吸附的构象之间的kβ″线能量转移,这随增加的种群而增加Cu +和Cu 2+阳离子的Cu-配体键数。通过分析的状态(PDOS)的部分密度这些结构中,Kβ“从A N 2S成Cu 1S跃迁发射线的结果,而Kβ 2,5-从混合N 2p和Cu 3d和4p态到Cu 1s芯孔的转变产生了发射线,其中Cu 4p态在该转变中起关键作用。进一步的PDOS分析显示了vtc-XES的化学敏感性,因为配体环境本质上是由配体2s状态的不同潜在结合能决定的。最后,我们将计算得到的XES结果与几种参考化合物的实测XES相比较,这似乎暗示了与文献中所建议的不同的赋值。因此,vtc-XES光谱是XANES的补充工具,非常适合在选择性催化还原NO或更一般而言,是工作催化剂中的金属离子。
更新日期:2017-11-15
中文翻译:

配体和氧化态对化合价X射线电子发射光谱的化学敏感性:多次H 2 O和NH 3吸附的Cu-SSZ-13的计算研究
价核X射线发射光谱(vtc-XES)是一种功能强大的实验工具,可以克服X射线吸收近边缘结构(XANES)测量对配体识别的灵敏度限制。为了进一步阐明该实验技术的敏感性,根据第一原理计算了在菱沸石结构(Cu-SSZ-13)中交换时相应的Cu阳离子发射光谱。通过比较吸附在Cu +(或Cu 2+)阳离子上的H 2 O和NH 3的vtc-XES光谱,我们发现kβ''线从Cu–O到Cu–N的连接发生了蓝移。此外,NH吸附3所导致更强的Kβ 2,5-线的强度要比对应的H 2 O的强度大。因此,可以区分Cu–O和Cu–N的不同vtc-XES发射线对H 2 O或NH 3的吸附。通过扫描多个H 2 O和NH 3吸附的Cu-SSZ-13结构的vtc-XES ,我们发现H 2 O和NH 3吸附的构象之间的kβ″线能量转移,这随增加的种群而增加Cu +和Cu 2+阳离子的Cu-配体键数。通过分析的状态(PDOS)的部分密度这些结构中,Kβ“从A N 2S成Cu 1S跃迁发射线的结果,而Kβ 2,5-从混合N 2p和Cu 3d和4p态到Cu 1s芯孔的转变产生了发射线,其中Cu 4p态在该转变中起关键作用。进一步的PDOS分析显示了vtc-XES的化学敏感性,因为配体环境本质上是由配体2s状态的不同潜在结合能决定的。最后,我们将计算得到的XES结果与几种参考化合物的实测XES相比较,这似乎暗示了与文献中所建议的不同的赋值。因此,vtc-XES光谱是XANES的补充工具,非常适合在选择性催化还原NO或更一般而言,是工作催化剂中的金属离子。











































 京公网安备 11010802027423号
京公网安备 11010802027423号