Journal of Power Sources ( IF 8.1 ) Pub Date : 2017-11-14 , DOI: 10.1016/j.jpowsour.2017.11.004 Xiao Tan , Rui Liu , Congxin Xie , Qiang Shen
|
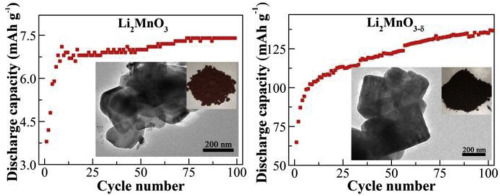
Lithium-rich manganese(IV) oxide Li2MnO3 has hardly any activity as the cathode active substance of lithium-ion batteries (LIBs) but its reversible capacity can be greatly improved by introducing oxygen deficiencies. After the solid-state heat treatment of nanocrystalline Li2MnO3 by sodium borohydride (NaBH4), the resulting Li2MnO3-δ crystallites comparatively acquire distinguishable appearances in color and shape and slight differences in surface composition and lattice structure. As a LIB cathode within the potential range of 2.5–4.7 V, at 20 mA g−1 pristine Li2MnO3 gives the specific discharge capacities of 3.3, 5.0 and 7.4 mAh·g−1 in the 1st, 10th and 100th cycles, while the derivative Li2MnO3-δ delivers the relatively high values of 64.8, 103.8 and 140.2 mAh·g−1 in the 1st, 10th and 120th cycles, respectively. Aside from the similar phenomenon of gradual electrochemical activation, substituting Li2MnO3-δ for Li2MnO3 means the great enhancements of charge-transfer ability and electrochemical performances. Especially, the cationic-anionic redox mechanisms of Li2MnO3 and Li2MnO3-δ are similar to each other, suggesting a possible solution to prepare high-performance xLi2MnO3-δ·(1-x)LiMO2 solid solutions for application purposes.
中文翻译:

从原始的Li 2 MnO 3获得的缺氧Li 2 MnO3 -δ的修饰结构特性和增强的电化学性能
富锂的锰(IV)氧化物Li 2 MnO 3作为锂离子电池(LIBs)的阴极活性物质几乎没有任何活性,但通过引入缺氧可以大大提高其可逆容量。在通过硼氢化钠(NaBH 4)对纳米晶态的Li 2 MnO 3进行固态热处理之后,所得的Li 2 MnO3 -δ微晶在颜色和形状上具有相对明显的外观,并且在表面组成和晶格结构上略有差异。作为LIB阴极,电位范围为2.5–4.7 V,在20 mA g -1时原始Li 2 MnO 3在第1个,第10个和第100个循环中给出的比放电容量分别为3.3、5.0和7.4 mAh·g -1,而派生的Li 2 MnO3 -δ提供相对较高的值64.8、103.8和140.2 mAh·g -1在第1个,第10个和第120个周期中。除了类似的逐渐发生电化学活化的现象外,用Li 2 MnO3 -δ代替Li 2 MnO 3意味着电荷转移能力和电化学性能的极大提高。尤其是Li 2 MnO 3和Li 2 MnO3 -δ的阳离子-阴离子氧化还原机理彼此相似,为制备用于应用目的的高性能x Li 2 MnO3 -δ ·(1- x)LiMO 2固溶体提供了一种可能的解决方案。











































 京公网安备 11010802027423号
京公网安备 11010802027423号