PLOS Medicine ( IF 10.5 ) Pub Date : 2017-11-14 , DOI: 10.1371/journal.pmed.1002435 Kenneth H. Mayer , Kelly E. Seaton , Yunda Huang , Nicole Grunenberg , Abby Isaacs , Mary Allen , Julie E. Ledgerwood , Ian Frank , Magdalena E. Sobieszczyk , Lindsey R. Baden , Benigno Rodriguez , Hong Van Tieu , Georgia D. Tomaras , Aaron Deal , Derrick Goodman , Robert T. Bailer , Guido Ferrari , Ryan Jensen , John Hural , Barney S. Graham , John R. Mascola , Lawrence Corey , David C. Montefiori , ,
|
Background
VRC01 is an HIV-1 CD4 binding site broadly neutralizing antibody (bnAb) that is active against a broad range of HIV-1 primary isolates in vitro and protects against simian-human immunodeficiency virus (SHIV) when delivered parenterally to nonhuman primates. It has been shown to be safe and well tolerated after short-term administration in humans; however, its clinical and functional activity after longer-term administration has not been previously assessed.
Methods and findings
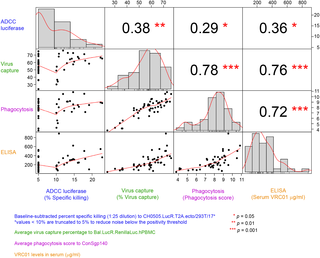
HIV Vaccine Trials Network (HVTN) 104 was designed to evaluate the safety and tolerability of multiple doses of VRC01 administered either subcutaneously or by intravenous (IV) infusion and to assess the pharmacokinetics and in vitro immunologic activity of the different dosing regimens. Additionally, this study aimed to assess the effect that the human body has on the functional activities of VRC01 as measured by several in vitro assays. Eighty-eight healthy, HIV-uninfected, low-risk participants were enrolled in 6 United States clinical research sites affiliated with the HVTN between September 9, 2014, and July 15, 2015. The median age of enrollees was 27 years (range, 18–50); 52% were White (non-Hispanic), 25% identified as Black (non-Hispanic), 11% were Hispanic, and 11% were non-Hispanic people of diverse origins. Participants were randomized to receive the following: a 40 mg/kg IV VRC01 loading dose followed by five 20 mg/kg IV VRC01 doses every 4 weeks (treatment group 1 [T1], n = 20); eleven 5 mg/kg subcutaneous (SC) VRC01 (treatment group 3 [T3], n = 20); placebo (placebo group 3 [P3], n = 4) doses every 2 weeks; or three 40 mg/kg IV VRC01 doses every 8 weeks (treatment group 2 [T2], n = 20). Treatment groups T4 and T5 (n = 12 each) received three 10 or 30 mg/kg IV VRC01 doses every 8 weeks, respectively. Participants were followed for 32 weeks after their first VRC01 administration and received a total of 249 IV infusions and 208 SC injections, with no serious adverse events, dose-limiting toxicities, nor evidence for anti-VRC01 antibodies observed. Serum VRC01 levels were detected through 12 weeks after final administration in all participants who received all scheduled doses. Mean peak serum VRC01 levels of 1,177 μg/ml (95% CI: 1,033, 1,340) and 420 μg/ml (95% CI: 356, 494) were achieved 1 hour after the IV infusion series of 30 mg/kg and 10 mg/kg doses, respectively. Mean trough levels at week 24 in the IV infusion series of 30 mg/kg and 10 mg/kg doses, respectively, were 16 μg/ml (95% CI: 10, 27) and 6 μg/ml (95% CI: 5, 9) levels, which neutralize a majority of circulating strains in vitro (50% inhibitory concentration [IC50] > 5 μg/ml). Post-infusion/injection serum VRC01 retained expected functional activity (virus neutralization, antibody-dependent cellular cytotoxicity, phagocytosis, and virion capture). The limitations of this study include the relatively small sample size of each VRC01 administration regimen and missing data from participants who were unable to complete all study visits.
Conclusions
VRC01 administered as either an IV infusion (10–40 mg/kg) given monthly or bimonthly, or as an SC injection (5 mg/kg) every 2 weeks, was found to be safe and well tolerated. In addition to maintaining drug concentrations consistent with neutralization of the majority of tested HIV strains, VRC01 concentrations from participants’ sera were found to avidly capture HIV virions and to mediate antibody-dependent cellular phagocytosis, suggesting a range of anti-HIV immunological activities, warranting further clinical trials.
Trial registration
Clinical Trials Registration: NCT02165267
中文翻译:

对未感染HIV的成年人进行多次静脉或皮下注射抗HIV单克隆抗体VRC01的安全性,药代动力学和免疫学活性:一项1期随机试验的结果
背景
VRC01是一种HIV-1 CD4结合位点广泛中和抗体(bnAb),在体外对多种HIV-1主要分离物具有活性,并在肠胃外递送给非人类灵长类动物时可抵抗猿猴-人类免疫缺陷病毒(SHIV)。已经证明,在人体中短期服用后,它是安全的并且具有良好的耐受性。但是,长期服用后其临床和功能活性尚未得到评估。
方法和发现
HIV疫苗试验网络(HVTN)104旨在评估皮下或静脉内(IV)输注多次剂量VRC01的安全性和耐受性,并评估不同给药方案的药代动力学和体外免疫活性。此外,这项研究旨在评估人体对VRC01的功能活性的影响,通过几种体外测定法进行测量。在2014年9月9日至2015年7月15日期间,有886名健康,未感染HIV的低风险参与者参加了HVTN附属的6个美国临床研究中心。入组者的年龄中位数为27岁(范围为18岁–50);白人(非西班牙裔)占52%,黑人(非西班牙裔)占25%,西班牙裔占11%,非裔西班牙裔占11%。n = 20);11个5 mg / kg皮下(SC)VRC01(治疗组3 [T3],n = 20);安慰剂(安慰剂组3 [P3],n = 4)每2周给药一次;或每8周3次40 mg / kg IV VRC01剂量(治疗组2 [T2],n = 20)。治疗组T4和T5(n每组= 12)分别每8周接受3次10或30 mg / kg静脉内VRC01剂量。首次服用VRC01后,对参与者进行了32周的随访,共进行了249次IV输注和208次SC注射,没有出现严重的不良事件,剂量限制性毒性,也未观察到抗VRC01抗体的证据。在接受所有预定剂量的所有受试者中,在最终用药后12周内检测到血清VRC01水平。静脉输注30 mg / kg和10 mg静脉注射1小时后,平均血清VRC01峰值峰值分别为1,177μg/ ml(95%CI:1,033,1,340)和420μg/ ml(95%CI:356,494) / kg剂量。静脉输注系列30 mg / kg和10 mg / kg剂量的第24周的平均谷值分别为16μg/ ml(95%CI:10,27)和6μg/ ml(95%CI:5) ,9)级,可以在体外中和大多数循环菌株(50%抑制浓度[IC50]> 5μg/ ml)。输注/注射后血清VRC01保留了预期的功能活性(病毒中和,抗体依赖性细胞的细胞毒性,吞噬作用和病毒体捕获)。该研究的局限性包括每种VRC01给药方案的样本量相对较小,以及无法完成所有研究访视的参与者缺少的数据。
结论
发现VRC01每月或每两个月静脉输注(10–40 mg / kg)或每两周一次SC注射(5 mg / kg)给药是安全且耐受性良好的。除了保持与大多数受测HIV菌株的中和一致的药物浓度外,还发现参与者血清中的VRC01浓度可狂热捕获HIV病毒粒子并介导抗体依赖性细胞吞噬作用,这表明一系列抗HIV免疫活性值得我们关注。进一步的临床试验。
试用注册
临床试验注册:NCT02165267











































 京公网安备 11010802027423号
京公网安备 11010802027423号