当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A pseudopericyclic [3,5]-sigmatropic rearrangement of a coumarin trichloroacetimidate derivative
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2017-10-27 00:00:00 , DOI: 10.1039/c7ob02335a Trideep Rajale 1, 2, 3, 4, 5 , Shikha Sharma 1, 2, 3, 4 , Daniel K. Unruh 1, 2, 3, 4 , Daniel A. Stroud 1, 2, 3, 4 , David M. Birney 1, 2, 3, 4
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2017-10-27 00:00:00 , DOI: 10.1039/c7ob02335a Trideep Rajale 1, 2, 3, 4, 5 , Shikha Sharma 1, 2, 3, 4 , Daniel K. Unruh 1, 2, 3, 4 , Daniel A. Stroud 1, 2, 3, 4 , David M. Birney 1, 2, 3, 4
Affiliation
|
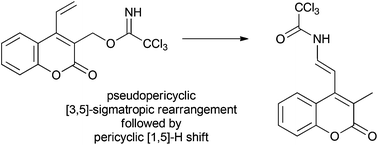
Thermolysis of a coumarin trichloroacetimidate yields a single rearrangement product. The proposed mechanism is a pseudopericyclic allowed (Woodward–Hoffman forbidden) [3,5]-sigmatropic rearrangement to form the corresponding amide followed by a sigmatropic [1,5]-hydrogen migration. Transition state calculations at the B3LYP/6-31G(d,p) level support this mechanism and suggest the selectivity is influenced by the stability of the intermediates.
中文翻译:

香豆素三氯乙酰亚氨酸酯衍生物的伪周边[3,5]-σ重排
香豆素三氯乙酰亚胺酸酯的热解产生单个重排产物。提出的机制是准周环允许的(伍德沃德-霍夫曼禁用)[3,5]-σ重排形成相应的酰胺,然后发生σ[1,5]-氢迁移。在B3LYP / 6-31G(d,p)水平上的过渡态计算支持该机制,并表明选择性受中间体稳定性的影响。
更新日期:2017-11-15
中文翻译:

香豆素三氯乙酰亚氨酸酯衍生物的伪周边[3,5]-σ重排
香豆素三氯乙酰亚胺酸酯的热解产生单个重排产物。提出的机制是准周环允许的(伍德沃德-霍夫曼禁用)[3,5]-σ重排形成相应的酰胺,然后发生σ[1,5]-氢迁移。在B3LYP / 6-31G(d,p)水平上的过渡态计算支持该机制,并表明选择性受中间体稳定性的影响。











































 京公网安备 11010802027423号
京公网安备 11010802027423号