当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Electrophilic activation of aminocarboxylic acid by phosphate ester promotes Friedel–Crafts acylation by overcoming charge–charge repulsion
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2017-10-26 00:00:00 , DOI: 10.1039/c7ob02158e Akinari Sumita 1, 2, 3, 4 , Yuko Otani 1, 2, 3, 4 , Tomohiko Ohwada 1, 2, 3, 4
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2017-10-26 00:00:00 , DOI: 10.1039/c7ob02158e Akinari Sumita 1, 2, 3, 4 , Yuko Otani 1, 2, 3, 4 , Tomohiko Ohwada 1, 2, 3, 4
Affiliation
|
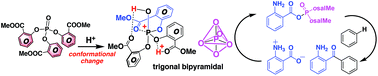
Friedel–Crafts acylation of aromatic compounds with aminocarboxylic acids proceeds efficiently in the presence of a tailored phosphate ester and a strong Brønsted acid, despite the strong charge–charge repulsion associated with acylium ion formation. Here, we investigate the mechanism of this electrophilic aromatic acylation reaction, focusing on how the aminocarboxylic acid is activated by the phosphate ester and how the charge–charge repulsion is overcome. In the first step of the reaction, an acyl phosphate is generated from the aminocarboxylic acid through the intervention of the phosphate ester, which possesses three methyl salicylate ester linkages. The o-methyl salicylates enhance the reactivity of the phosphate ester via a protonation-induced conformational change, thereby overwhelming the charge–charge repulsion associated with the acylium ion formation. Weakening of the resonance interaction in the C(O)–O(P) bond by the lone-pair electrons of the ether oxygen atom of the carboxylic acid functionality contributes to the rapid formation of the acylium ion. Thus, our results show that the formation of aromatic ketones from various carboxylic acids proceeds because the strong leaving ability of the acyl phosphate overwhelms the charge–charge repulsion associated with the formation of the acylium ion. This information will be helpful to improve the design of tailored phosphate reagents.
中文翻译:

磷酸酯对氨基羧酸的亲电活化通过克服电荷-电荷排斥作用,促进了弗里德-克拉夫茨的酰化反应
尽管存在与离子形成相关的强电荷-电荷排斥作用,但在特制的磷酸酯和强布朗斯台德酸存在下,芳族化合物与氨基羧酸的Friedel-Crafts酰化反应仍能有效进行。在这里,我们研究了这种亲电芳族酰化反应的机理,重点研究了氨基羧酸如何被磷酸酯活化以及如何克服电荷-电荷排斥。在反应的第一步中,通过具有三个水杨酸甲酯酯键的磷酸酯的干预,由氨基羧酸生成酰基磷酸酯。所述ø -甲基水杨酸盐增强磷酸酯的反应性通过质子化引起的构象变化,从而压倒了与acy离子形成相关的电荷-电荷排斥。羧酸官能团的醚氧原子的孤对电子削弱了C(O)–O(P)键中的共振相互作用,从而促进了lium离子的迅速形成。因此,我们的结果表明,由多种羧酸形成芳香族酮的过程继续进行,因为酰基磷酸酯的强离去能力压倒了与with离子形成相关的电荷-电荷排斥。此信息将有助于改进定制的磷酸盐试剂的设计。
更新日期:2017-11-15
中文翻译:

磷酸酯对氨基羧酸的亲电活化通过克服电荷-电荷排斥作用,促进了弗里德-克拉夫茨的酰化反应
尽管存在与离子形成相关的强电荷-电荷排斥作用,但在特制的磷酸酯和强布朗斯台德酸存在下,芳族化合物与氨基羧酸的Friedel-Crafts酰化反应仍能有效进行。在这里,我们研究了这种亲电芳族酰化反应的机理,重点研究了氨基羧酸如何被磷酸酯活化以及如何克服电荷-电荷排斥。在反应的第一步中,通过具有三个水杨酸甲酯酯键的磷酸酯的干预,由氨基羧酸生成酰基磷酸酯。所述ø -甲基水杨酸盐增强磷酸酯的反应性通过质子化引起的构象变化,从而压倒了与acy离子形成相关的电荷-电荷排斥。羧酸官能团的醚氧原子的孤对电子削弱了C(O)–O(P)键中的共振相互作用,从而促进了lium离子的迅速形成。因此,我们的结果表明,由多种羧酸形成芳香族酮的过程继续进行,因为酰基磷酸酯的强离去能力压倒了与with离子形成相关的电荷-电荷排斥。此信息将有助于改进定制的磷酸盐试剂的设计。











































 京公网安备 11010802027423号
京公网安备 11010802027423号