当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Photochemical and oxidative cyclisation of tetraphenylpyrroles
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2017-10-25 00:00:00 , DOI: 10.1039/c7ob02537h Jayne L. Ferguson 1, 2, 3, 4 , Marie A. Squire 1, 2, 3, 4 , Christopher M. Fitchett 1, 2, 3, 4
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2017-10-25 00:00:00 , DOI: 10.1039/c7ob02537h Jayne L. Ferguson 1, 2, 3, 4 , Marie A. Squire 1, 2, 3, 4 , Christopher M. Fitchett 1, 2, 3, 4
Affiliation
|
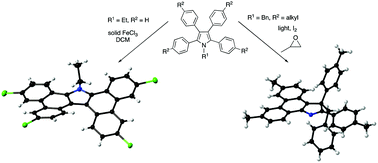
The photochemical and oxidative cyclodehydrogenation reactions of tetraphenylpyrroles act in a complementary fashion for the cyclisation of N-ethyl and N-benzyl derivatives. In the case of the former, a doubly cyclised product was isolated from cyclisation with solid FeCl3, while the latter gives a rearranged 3H-pyrrole upon irradiation.
中文翻译:

四苯基吡咯的光化学和氧化环化
四苯基吡咯的光化学和氧化环脱氢反应以互补的方式作用于N-乙基和N-苄基衍生物的环化。在前者的情况下,从固体FeCl 3的环化中分离出双环化产物,而后者在辐照时给出了重排的3 H-吡咯。
更新日期:2017-11-15
中文翻译:

四苯基吡咯的光化学和氧化环化
四苯基吡咯的光化学和氧化环脱氢反应以互补的方式作用于N-乙基和N-苄基衍生物的环化。在前者的情况下,从固体FeCl 3的环化中分离出双环化产物,而后者在辐照时给出了重排的3 H-吡咯。











































 京公网安备 11010802027423号
京公网安备 11010802027423号