当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Total syntheses of all tri-oxygenated 16-phytoprostane classes via a common precursor constructed by oxidative cyclization and alkyl–alkyl coupling reactions as the key steps
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2017-10-24 00:00:00 , DOI: 10.1039/c7ob02505j Jakub Smrček 1, 2, 3, 4 , Radek Pohl 1, 2, 3, 4 , Ullrich Jahn 1, 2, 3, 4
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2017-10-24 00:00:00 , DOI: 10.1039/c7ob02505j Jakub Smrček 1, 2, 3, 4 , Radek Pohl 1, 2, 3, 4 , Ullrich Jahn 1, 2, 3, 4
Affiliation
|
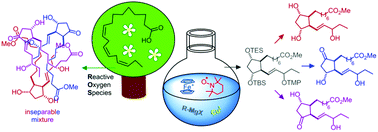
A unified strategy for the total synthesis of the methyl esters of all phytoprostane (PhytoP) classes bearing two ring-oxygen atoms based on an orthogonally protected common precursor is described. Racemic 16-F1t-, 16-E1-PhytoP and their C-16 epimers, which also occur as racemates in Nature, were successfully obtained. The first total synthesis of very sensitive 16-D1t-PhytoP succeeded, however, it quickly isomerized to more stable, but so far also unknown Δ13-16-D1t-PhytoP, which may serve as a more reliable biomarker for D-type PhytoP. The dioxygenated cyclopentane ring carrying the ω-chain with the oxygen functionality in the 16-position was approached by a radical oxidative cyclization mediated by ferrocenium hexafluorophosphate and TEMPO. The α-chain was introduced by a new copper-catalyzed alkyl–alkyl coupling of a 6-heptenyl Grignard reagent with a functionalized cyclopentylmethyl triflate.
中文翻译:

所有三氧合16 phytoprostane类的全合成通过一个共同的前体通过氧化环化和烷基-烷基偶联反应作为关键步骤构建
描述了一种基于正交保护的共同前体对具有两个环氧原子的所有植物前列腺素(PhytoP)类甲酯进行全合成的统一策略。成功获得了消旋体16-F 1t-,16-E 1 -PhytoP及其C-16差向异构体,它们在大自然中也作为消旋体存在。非常敏感16 d的首次全合成1吨-PhytoP成功,然而,它迅速异构化为较稳定,但到目前为止还未知Δ 13 -16-d 1吨-PhytoP,可以用作D型PhytoP的更可靠的生物标记。六氟磷酸铁和TEMPO介导的自由基氧化环化作用使带有ω链且在16位具有氧官能团的双加氧环戊烷环接近。通过6-庚烯基格氏试剂与功能化的环戊基甲基三氟甲磺酸酯的新的铜催化的烷基-烷基偶联引入α-链。
更新日期:2017-11-15
中文翻译:

所有三氧合16 phytoprostane类的全合成通过一个共同的前体通过氧化环化和烷基-烷基偶联反应作为关键步骤构建
描述了一种基于正交保护的共同前体对具有两个环氧原子的所有植物前列腺素(PhytoP)类甲酯进行全合成的统一策略。成功获得了消旋体16-F 1t-,16-E 1 -PhytoP及其C-16差向异构体,它们在大自然中也作为消旋体存在。非常敏感16 d的首次全合成1吨-PhytoP成功,然而,它迅速异构化为较稳定,但到目前为止还未知Δ 13 -16-d 1吨-PhytoP,可以用作D型PhytoP的更可靠的生物标记。六氟磷酸铁和TEMPO介导的自由基氧化环化作用使带有ω链且在16位具有氧官能团的双加氧环戊烷环接近。通过6-庚烯基格氏试剂与功能化的环戊基甲基三氟甲磺酸酯的新的铜催化的烷基-烷基偶联引入α-链。











































 京公网安备 11010802027423号
京公网安备 11010802027423号