当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Chemoselective N-arylation of aminobenzamides via copper catalysed Chan–Evans–Lam reactions
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2017-10-23 00:00:00 , DOI: 10.1039/c7ob02491f Shuai Liu 1, 2, 3, 4 , Weisai Zu 1, 2, 3, 4 , Jinli Zhang 1, 2, 3, 4, 5 , Liang Xu 1, 2, 3, 4
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2017-10-23 00:00:00 , DOI: 10.1039/c7ob02491f Shuai Liu 1, 2, 3, 4 , Weisai Zu 1, 2, 3, 4 , Jinli Zhang 1, 2, 3, 4, 5 , Liang Xu 1, 2, 3, 4
Affiliation
|
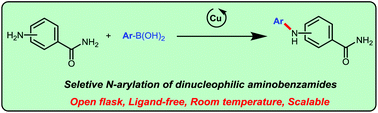
Chemoselective N-arylation of unprotected aminobenzamides was achieved via Cu-catalysed Chan–Evans–Lam cross-coupling with aryl boronic acids for the first time. Simple copper catalysts enable the selective arylation of amino groups in ortho/meta/para-aminobenzamides under open-flask conditions. The reactions were scalable and compatible with a wide range of functional groups.
中文翻译:

化学选择性Ñ氨基苯甲酰胺的-arylation通过铜催化的赞埃文斯-榄反应
未保护的氨基苯甲酰胺的化学选择性N-芳基化是通过Cu催化的Chan-Evans-Lam与芳基硼酸的首次交叉偶联而实现的。简单的铜催化剂可以在开瓶条件下使邻/间/对-氨基苯甲酰胺中的氨基选择性芳基化。反应是可扩展的,并且与多种官能团相容。
更新日期:2017-11-15
中文翻译:

化学选择性Ñ氨基苯甲酰胺的-arylation通过铜催化的赞埃文斯-榄反应
未保护的氨基苯甲酰胺的化学选择性N-芳基化是通过Cu催化的Chan-Evans-Lam与芳基硼酸的首次交叉偶联而实现的。简单的铜催化剂可以在开瓶条件下使邻/间/对-氨基苯甲酰胺中的氨基选择性芳基化。反应是可扩展的,并且与多种官能团相容。









































 京公网安备 11010802027423号
京公网安备 11010802027423号