当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Facile access to functionalized indenes and fused quinolines by regioselective 5-enolexo-dig Michael addition and cyclization reactions
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2017-10-13 00:00:00 , DOI: 10.1039/c7ob02498c Tohasib Yusub Chaudhari 1, 2, 3, 4 , Sandeep K. Ginotra 4, 5, 6, 7 , Vibha Tandon 1, 2, 3, 4
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2017-10-13 00:00:00 , DOI: 10.1039/c7ob02498c Tohasib Yusub Chaudhari 1, 2, 3, 4 , Sandeep K. Ginotra 4, 5, 6, 7 , Vibha Tandon 1, 2, 3, 4
Affiliation
|
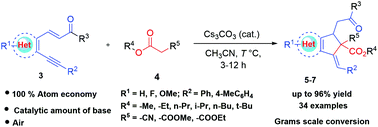
Herein we report a facile approach to synthesise multi-substituted indenes and cyclopenta[b]quinolines under mild conditions. The reaction proceeds via Michael addition between commercially available cyanoacetate/malonic esters and α,β-unsaturated ketones. The synthetic methodology involves enolate mediated regio- and stereoselective intramolecular 5-enolexo-dig cyclization promoted by a catalytic base. The products stereoselectively form cis-isomers for indenes and trans-isomers for cyclopenta[b]quinolines, albeit with the presence of steric hindrance at a quaternary carbon substituted by active methylene compounds. The reaction pathway was investigated by isolating the reaction intermediate. This synthetic transformation was achieved with various aromatic and heteroaromatic Michael acceptors and the desired products were obtained in high to excellent yields. The reaction is scalable up to gram level with only 10 mol% of base.
中文翻译:

通过区域选择性5-烯属-dig迈克尔加成反应和环化反应可轻松获得官能化的茚满和稠合的喹啉
在这里,我们报道了一种在温和条件下合成多取代的茚满和环戊[ b ]喹啉的简便方法。反应通过可商购的氰基乙酸酯/丙二酸酯与α,β-不饱和酮之间的迈克尔加成进行。合成方法涉及由催化碱促进的烯醇盐介导的区域和立体选择性分子内5-烯醇式-dig环化。该产物立体选择性地形成环戊烯的顺式异构体和反式异构体[ b]喹啉,尽管在被活性亚甲基化合物取代的季碳上存在位阻。通过分离反应中间体来研究反应途径。用各种芳族和杂芳族迈克尔受体实现了这种合成转化,并且以高至优异的产率获得了所需的产物。仅需10摩尔%的碱,该反应即可扩展至克级。
更新日期:2017-11-15
中文翻译:

通过区域选择性5-烯属-dig迈克尔加成反应和环化反应可轻松获得官能化的茚满和稠合的喹啉
在这里,我们报道了一种在温和条件下合成多取代的茚满和环戊[ b ]喹啉的简便方法。反应通过可商购的氰基乙酸酯/丙二酸酯与α,β-不饱和酮之间的迈克尔加成进行。合成方法涉及由催化碱促进的烯醇盐介导的区域和立体选择性分子内5-烯醇式-dig环化。该产物立体选择性地形成环戊烯的顺式异构体和反式异构体[ b]喹啉,尽管在被活性亚甲基化合物取代的季碳上存在位阻。通过分离反应中间体来研究反应途径。用各种芳族和杂芳族迈克尔受体实现了这种合成转化,并且以高至优异的产率获得了所需的产物。仅需10摩尔%的碱,该反应即可扩展至克级。









































 京公网安备 11010802027423号
京公网安备 11010802027423号