当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Differentiation of enantiomeric anions by NMR spectroscopy with chiral bisurea receptors
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2017-11-06 00:00:00 , DOI: 10.1039/c7ob02318a Suguru Ito 1, 2, 3, 4, 5 , Manami Okuno 1, 2, 3, 4, 5 , Masatoshi Asami 1, 2, 3, 4, 5
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2017-11-06 00:00:00 , DOI: 10.1039/c7ob02318a Suguru Ito 1, 2, 3, 4, 5 , Manami Okuno 1, 2, 3, 4, 5 , Masatoshi Asami 1, 2, 3, 4, 5
Affiliation
|
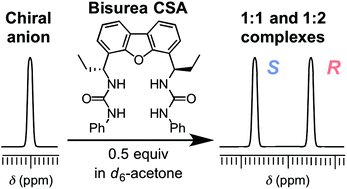
Chiral anionic species are ubiquitous and play important roles in biological systems. Despite the recent advancements in synthetic anion receptors bearing urea functionalities, urea-based chiral solvating agents (CSAs) that can separate the NMR signals of racemic anions remain limited. Herein, three dibenzofuran-based C2-symmetric chiral bisureas were synthesized from the reaction of (R,R)-4,6-bis(1-aminopropyl)dibenzo[b,d]furan with phenyl isocyanate, phenyl thioisocyanate, or tosyl isocyanate. The chiral anion recognition properties of these bisureas were examined by 1H NMR spectroscopy using DL-tetrabutylammonium mandelate (TBAM) as a model substrate. A clear baseline separation of the enantiomeric signals of the benzylic proton of TBAM was achieved upon mixing with 0.5 equivalents of bis(phenylurea). In contrast to previous urea-based chiral anion receptors that differentiate the enantiomers of chiral anions by forming 1 : 1 host–guest complexes, a high chiral recognition ability of chiral bis(phenylurea) was achieved owing to the generation of an equilibrium between free guests, 1 : 1 host–guest complexes, and 1 : 2 host–guest complexes. Chiral bis(phenylurea) was also successfully employed in the separation of the enantiomeric 1H NMR signals of various racemic anions.
中文翻译:

NMR光谱与手性双苏脲受体的对映体阴离子的区分
手性阴离子种类无处不在,并在生物系统中起重要作用。尽管具有尿素官能团的合成阴离子受体的最新进展,可分离外消旋阴离子的NMR信号的基于尿素的手性溶剂化剂(CSA)仍然受到限制。在此,由(R,R)-4,6-双(1-氨基丙基)二苯并[ b,d ]呋喃与异氰酸苯酯,苯基硫代异氰酸酯或甲苯磺酰基的反应合成了三种基于二苯并呋喃的C 2对称手性双偶氮基。异氰酸酯。通过使用DL的1 H NMR光谱检查了这些Bisureas的手性阴离子识别特性-扁桃酸四丁铵(TBAM)作为模型底物。与0.5当量的双(苯基脲)混合后,可实现TBAM苄基质子对映体信号的清晰基线分离。与以前的尿素基手性阴离子受体通过形成1:1主体-客体复合物来区分手性阴离子的对映异构体相反,由于游离客体之间的平衡,手性双(苯基脲)具有很高的手性识别能力。 ,1:1主客复合体和1:2主客复合体。手性双(苯基脲)也已成功用于各种外消旋阴离子的对映异构体1 H NMR信号的分离。
更新日期:2017-11-15
中文翻译:

NMR光谱与手性双苏脲受体的对映体阴离子的区分
手性阴离子种类无处不在,并在生物系统中起重要作用。尽管具有尿素官能团的合成阴离子受体的最新进展,可分离外消旋阴离子的NMR信号的基于尿素的手性溶剂化剂(CSA)仍然受到限制。在此,由(R,R)-4,6-双(1-氨基丙基)二苯并[ b,d ]呋喃与异氰酸苯酯,苯基硫代异氰酸酯或甲苯磺酰基的反应合成了三种基于二苯并呋喃的C 2对称手性双偶氮基。异氰酸酯。通过使用DL的1 H NMR光谱检查了这些Bisureas的手性阴离子识别特性-扁桃酸四丁铵(TBAM)作为模型底物。与0.5当量的双(苯基脲)混合后,可实现TBAM苄基质子对映体信号的清晰基线分离。与以前的尿素基手性阴离子受体通过形成1:1主体-客体复合物来区分手性阴离子的对映异构体相反,由于游离客体之间的平衡,手性双(苯基脲)具有很高的手性识别能力。 ,1:1主客复合体和1:2主客复合体。手性双(苯基脲)也已成功用于各种外消旋阴离子的对映异构体1 H NMR信号的分离。









































 京公网安备 11010802027423号
京公网安备 11010802027423号