当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Mononuclear Iron-Dependent Methyltransferase Catalyzes Initial Steps in Assembly of the Apratoxin A Polyketide Starter Unit
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2017-11-14 00:00:00 , DOI: 10.1021/acschembio.7b00746 Meredith A. Skiba 1, 2 , Andrew P. Sikkema 1, 2 , Nathan A. Moss 3 , Collin L. Tran 1 , Rebecca M. Sturgis 1 , Lena Gerwick 3 , William H. Gerwick 3, 4 , David H. Sherman 1, 5, 6, 7 , Janet L. Smith 1, 2
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2017-11-14 00:00:00 , DOI: 10.1021/acschembio.7b00746 Meredith A. Skiba 1, 2 , Andrew P. Sikkema 1, 2 , Nathan A. Moss 3 , Collin L. Tran 1 , Rebecca M. Sturgis 1 , Lena Gerwick 3 , William H. Gerwick 3, 4 , David H. Sherman 1, 5, 6, 7 , Janet L. Smith 1, 2
Affiliation
|
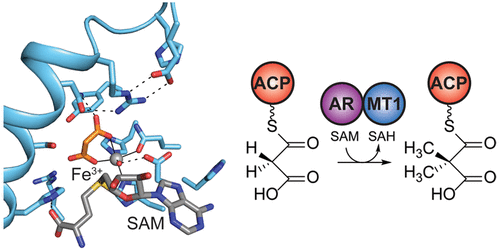
Natural product biosynthetic pathways contain a plethora of enzymatic tools to carry out difficult biosynthetic transformations. Here, we discover an unusual mononuclear iron-dependent methyltransferase that acts in the initiation steps of apratoxin A biosynthesis (AprA MT1). Fe3+-replete AprA MT1 catalyzes one or two methyl transfer reactions on the substrate malonyl-ACP (acyl carrier protein), whereas Co2+, Fe2+, Mn2+, and Ni2+ support only a single methyl transfer. MT1 homologues exist within the “GNAT” (GCN5-related N-acetyltransferase) loading modules of several modular biosynthetic pathways with propionyl, isobutyryl, or pivaloyl starter units. GNAT domains are thought to catalyze decarboxylation of malonyl-CoA and acetyl transfer to a carrier protein. In AprA, the GNAT domain lacks both decarboxylation and acyl transfer activity. A crystal structure of the AprA MT1-GNAT di-domain with bound Mn2+, malonate, and the methyl donor S-adenosylmethionine (SAM) reveals that the malonyl substrate is a bidentate metal ligand, indicating that the metal acts as a Lewis acid to promote methylation of the malonyl α-carbon. The GNAT domain is truncated relative to functional homologues. These results afford an expanded understanding of MT1-GNAT structure and activity and permit the functional annotation of homologous GNAT loading modules both with and without methyltransferases, additionally revealing their rapid evolutionary adaptation in different biosynthetic contexts.
中文翻译:

单核铁依赖性甲基转移酶催化Apratoxin A聚酮化合物启动器单元组装的初始步骤。
天然产物的生物合成途径包含大量的酶促工具,可以进行困难的生物合成转化。在这里,我们发现了一种异常的单核铁依赖性甲基转移酶,该酶在pratoxin A生物合成(AprA MT1)的起始步骤中起作用。富含Fe 3+的AprA MT1催化底物丙二酰ACP(酰基载体蛋白)上的一个或两个甲基转移反应,而Co 2 +,Fe 2 +,Mn 2+和Ni 2+仅支持一次甲基转移。MT1同源物存在于“ GNAT”(与GCN5相关的N-乙酰转移酶)加载带有丙酰基,异丁酰基或新戊酰基起始剂单元的几种模块化生物合成途径的模块。GNAT结构域被认为可催化丙二酰辅酶A的脱羧反应和乙酰转移到载体蛋白上。在AprA中,GNAT域既缺乏脱羧作用又没有酰基转移活性。结合了Mn 2+,丙二酸酯和甲基供体S的AprA MT1-GNAT双结构域的晶体结构-腺苷甲硫氨酸(SAM)揭示丙二酸底物是双齿金属配体,表明该金属充当路易斯酸以促进丙二酸α-碳的甲基化。GNAT域相对于功能同源物被截短。这些结果提供了对MT1-GNAT结构和活性的扩展理解,并允许具有和不具有甲基转移酶的同源GNAT加载模块的功能注释,另外揭示了它们在不同生物合成环境中的快速进化适应性。
更新日期:2017-11-15
中文翻译:

单核铁依赖性甲基转移酶催化Apratoxin A聚酮化合物启动器单元组装的初始步骤。
天然产物的生物合成途径包含大量的酶促工具,可以进行困难的生物合成转化。在这里,我们发现了一种异常的单核铁依赖性甲基转移酶,该酶在pratoxin A生物合成(AprA MT1)的起始步骤中起作用。富含Fe 3+的AprA MT1催化底物丙二酰ACP(酰基载体蛋白)上的一个或两个甲基转移反应,而Co 2 +,Fe 2 +,Mn 2+和Ni 2+仅支持一次甲基转移。MT1同源物存在于“ GNAT”(与GCN5相关的N-乙酰转移酶)加载带有丙酰基,异丁酰基或新戊酰基起始剂单元的几种模块化生物合成途径的模块。GNAT结构域被认为可催化丙二酰辅酶A的脱羧反应和乙酰转移到载体蛋白上。在AprA中,GNAT域既缺乏脱羧作用又没有酰基转移活性。结合了Mn 2+,丙二酸酯和甲基供体S的AprA MT1-GNAT双结构域的晶体结构-腺苷甲硫氨酸(SAM)揭示丙二酸底物是双齿金属配体,表明该金属充当路易斯酸以促进丙二酸α-碳的甲基化。GNAT域相对于功能同源物被截短。这些结果提供了对MT1-GNAT结构和活性的扩展理解,并允许具有和不具有甲基转移酶的同源GNAT加载模块的功能注释,另外揭示了它们在不同生物合成环境中的快速进化适应性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号