当前位置:
X-MOL 学术
›
ACS Sustain. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Furylated Flavonoids: Fully Biobased Building Blocks Produced by Condensed Tannins Depolymerization
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2017-11-14 00:00:00 , DOI: 10.1021/acssuschemeng.7b03409 Laurent Rouméas 1, 2, 3 , Guillaume Billerach 1 , Chahinez Aouf 1 , Éric Dubreucq 2 , Hélène Fulcrand 1
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2017-11-14 00:00:00 , DOI: 10.1021/acssuschemeng.7b03409 Laurent Rouméas 1, 2, 3 , Guillaume Billerach 1 , Chahinez Aouf 1 , Éric Dubreucq 2 , Hélène Fulcrand 1
Affiliation
|
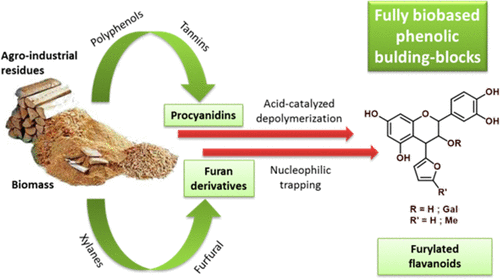
An original method has been set up to produce fully biobased phenolic building blocks from condensed tannins, largely available from agroindustrial residues or wood industry coproducts. The acid-catalyzed depolymerization of condensed tannins in the presence of furan or sylvan in mild conditions (30–40 °C, 0.1 M HCl) gives the corresponding furylated flavonoids with high yields. The reaction was more efficient with sylvan than with the less nucleophilic furan. A key feature of the products is the high stability of the flavanyl to furyl C–C linkage compared to the thioether bond obtained by the classical thiolysis, which makes them promising platform molecules for further functionalization, including in alkaline conditions. The simplicity of the process makes it easy to scale-up, and the reaction can be carried out on raw plant materials directly. In accordance with green chemistry concepts, solvents and excess reagents can readily be recovered by distillation and recycled.
中文翻译:

糠醛黄酮类化合物:缩合单宁解聚产生的完全基于生物的构建基块
已经建立了一种原始方法,该方法可以从浓缩的单宁酸生产完全基于生物的酚醛建筑材料,而该技术很大程度上可以从农用工业残留物或木材工业副产品中获得。在温和的条件下(30–40°C,0.1 M HCl),在呋喃或森林中存在的条件下,缩合单宁酸的酸催化解聚反应可以高产率地得到相应的糠醛化类黄酮。与低亲核呋喃相比,使用西尔万反应更有效。与经典硫解法获得的硫醚键相比,该产品的主要特点是黄烷酰基与呋喃基C–C键具有很高的稳定性,这使其成为有望在包括碱性条件下进一步官能化的分子平台。该方法的简单性使其易于扩大规模,并且该反应可以直接在植物原料上进行。
更新日期:2017-11-14
中文翻译:

糠醛黄酮类化合物:缩合单宁解聚产生的完全基于生物的构建基块
已经建立了一种原始方法,该方法可以从浓缩的单宁酸生产完全基于生物的酚醛建筑材料,而该技术很大程度上可以从农用工业残留物或木材工业副产品中获得。在温和的条件下(30–40°C,0.1 M HCl),在呋喃或森林中存在的条件下,缩合单宁酸的酸催化解聚反应可以高产率地得到相应的糠醛化类黄酮。与低亲核呋喃相比,使用西尔万反应更有效。与经典硫解法获得的硫醚键相比,该产品的主要特点是黄烷酰基与呋喃基C–C键具有很高的稳定性,这使其成为有望在包括碱性条件下进一步官能化的分子平台。该方法的简单性使其易于扩大规模,并且该反应可以直接在植物原料上进行。











































 京公网安备 11010802027423号
京公网安备 11010802027423号