Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Relationship of C-reactive protein reduction to cardiovascular event reduction following treatment with canakinumab: a secondary analysis from the CANTOS randomised controlled trial.
The Lancet ( IF 98.4 ) Pub Date : 2017-11-13 , DOI: 10.1016/s0140-6736(17)32814-3 Paul M Ridker 1 , Jean G MacFadyen 2 , Brendan M Everett 1 , Peter Libby 3 , Tom Thuren 4 , Robert J Glynn 2 ,
The Lancet ( IF 98.4 ) Pub Date : 2017-11-13 , DOI: 10.1016/s0140-6736(17)32814-3 Paul M Ridker 1 , Jean G MacFadyen 2 , Brendan M Everett 1 , Peter Libby 3 , Tom Thuren 4 , Robert J Glynn 2 ,
Affiliation
|
BACKGROUND
Canakinumab, a monoclonal antibody targeting interleukin-1β, reduces inflammation and cardiovascular event rates with no effect on lipid concentrations. However, it is uncertain which patient groups benefit the most from treatment and whether reductions in the inflammatory biomarker high-sensitivity C-reactive protein (hsCRP) correlate with clinical benefits for individual patients.
METHODS
The Canakinumab Anti-Inflammatory Thrombosis Outcomes Study (CANTOS) used computer-generated codes to randomly allocate 10 061 men and women with a history of myocardial infarction to placebo or one of three doses of canakinumab (50 mg, 150 mg, or 300 mg) given subcutaneously once every 3 months. In a prespecified secondary analysis designed to address the relationship of hsCRP reduction to event reduction in CANTOS, we evaluated the effects of canakinumab on rates of major adverse cardiovascular events, cardiovascular mortality, and all-cause mortality according to on-treatment concentrations of hsCRP. We used multivariable modelling to adjust for baseline factors associated with achieved hsCRP and multiple sensitivity analyses to address the magnitude of residual confounding. The median follow-up was 3·7 years. The trial is registered with ClinicalTrials.gov, number NCT01327846.
FINDINGS
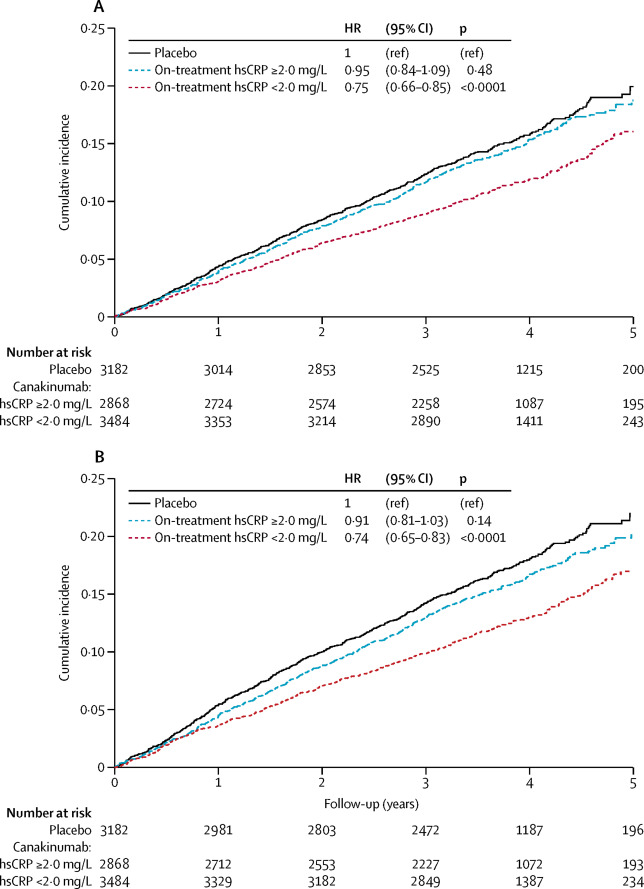
Baseline clinical characteristics did not define patient groups with greater or lesser cardiovascular benefits when treated with canakinumab. However, trial participants allocated to canakinumab who achieved hsCRP concentrations less than 2 mg/L had a 25% reduction in major adverse cardiovascular events (multivariable adjusted hazard ratio [HRadj]=0·75, 95% CI 0·66-0·85, p<0·0001), whereas no significant benefit was observed among those with on-treatment hsCRP concentrations of 2 mg/L or above (HRadj=0·90, 0·79-1·02, p=0·11). For those treated with canakinumab who achieved on-treatment hsCRP concentrations less than 2 mg/L, cardiovascular mortality (HRadj=0·69, 95% CI 0·56-0·85, p=0·0004) and all-cause mortality (HRadj=0·69, 0·58-0·81, p<0·0001) were both reduced by 31%, whereas no significant reduction in these endpoints was observed among those treated with canakinumab who achieved hsCRP concentrations of 2 mg/L or above. Similar differential effects were found in analyses of the trial prespecified secondary cardiovascular endpoint (which additionally included hospitalisation for unstable angina requiring unplanned revascularisation) and in sensitivity analyses alternatively based on median reductions in hsCRP, on 50% or greater reductions in hsCRP, on the median percent reduction in hsCRP, in dose-specific analyses, and in analyses using a causal inference approach to estimate the effect of treatment among individuals who would achieve a targeted hsCRP concentration.
INTERPRETATION
The magnitude of hsCRP reduction following a single dose of canakinumab might provide a simple clinical method to identify individuals most likely to accrue the largest benefit from continued treatment. These data further suggest that lower is better for inflammation reduction with canakinumab.
FUNDING
Novartis Pharmaceuticals.
中文翻译:

canakinumab治疗后C反应蛋白减少与心血管事件减少的关系:来自CANTOS随机对照试验的次要分析。
背景技术Canakinumab是一种靶向白介素1β的单克隆抗体,可降低炎症和心血管事件发生率,而对脂质浓度没有影响。但是,尚不确定哪些患者组从治疗中受益最大,以及炎症生物标志物高敏C反应蛋白(hsCRP)的降低是否与个别患者的临床获益相关。方法Canakinumab抗炎性血栓形成结果研究(CANTOS)使用计算机生成的代码将10 061名有心肌梗塞病史的男性和女性随机分配给安慰剂或三剂canakinumab(50 mg,150 mg或300 mg)之一),每3个月一次皮下注射。在旨在解决hsCRP减少与CANTOS中事件减少之间的关系的预先指定的二级分析中,我们根据hsCRP的治疗浓度评估了canakinumab对主要不良心血管事件发生率,心血管死亡率和全因死亡率的影响。我们使用多变量建模来调整与已实现的hsCRP相关的基线因素,并使用多重敏感性分析来解决残余混杂的程度。中位随访时间为3·7年。该试验已在ClinicalTrials.gov上注册,编号为NCT01327846。研究结果基线临床特征未定义接受卡那基单抗治疗时具有或多或少的心血管益处的患者组。但是,分配给canakinumab且hsCRP浓度低于2 mg / L的试验参与者的主要不良心血管事件减少了25%(多变量调整的危险比[HRadj] = 0·75,95%CI 0·66-0·85 ,p <0·0001),而在治疗中hsCRP浓度为2 mg / L或更高的患者中未观察到显着获益(HRadj = 0·90,0·79-1·02,p = 0·11)。对于使用卡那基单抗治疗且治疗中hsCRP浓度低于2 mg / L的患者,心血管疾病死亡率(HRadj = 0·69,95%CI 0·56-0·85,p = 0·0004)和全因死亡率(HRadj = 0·69,0·58-0·81,p <0·0001)均降低了31%,而接受canakinumab治疗且hsCRP浓度达到2 mg / L或以上。在试验中预先设定的次要心血管终点(包括因不稳定心绞痛而需要计划外血运重建的住院治疗)的分析中,或者在基于hsCRP降低的中位数,hsCRP降低的50%或更高的敏感性分析中,也发现了类似的差异作用。 hsCRP降低百分比,剂量特异性分析以及使用因果推论方法进行的分析,以评估达到目标hsCRP浓度的个体的治疗效果。解释单次使用canakinumab后hsCRP降低的幅度可能提供一种简单的临床方法,以鉴定最有可能从持续治疗中获得最大益处的个体。这些数据进一步表明,较低的剂量对使用canakinumab减轻炎症效果更好。资金诺华制药。
更新日期:2018-01-26
中文翻译:

canakinumab治疗后C反应蛋白减少与心血管事件减少的关系:来自CANTOS随机对照试验的次要分析。
背景技术Canakinumab是一种靶向白介素1β的单克隆抗体,可降低炎症和心血管事件发生率,而对脂质浓度没有影响。但是,尚不确定哪些患者组从治疗中受益最大,以及炎症生物标志物高敏C反应蛋白(hsCRP)的降低是否与个别患者的临床获益相关。方法Canakinumab抗炎性血栓形成结果研究(CANTOS)使用计算机生成的代码将10 061名有心肌梗塞病史的男性和女性随机分配给安慰剂或三剂canakinumab(50 mg,150 mg或300 mg)之一),每3个月一次皮下注射。在旨在解决hsCRP减少与CANTOS中事件减少之间的关系的预先指定的二级分析中,我们根据hsCRP的治疗浓度评估了canakinumab对主要不良心血管事件发生率,心血管死亡率和全因死亡率的影响。我们使用多变量建模来调整与已实现的hsCRP相关的基线因素,并使用多重敏感性分析来解决残余混杂的程度。中位随访时间为3·7年。该试验已在ClinicalTrials.gov上注册,编号为NCT01327846。研究结果基线临床特征未定义接受卡那基单抗治疗时具有或多或少的心血管益处的患者组。但是,分配给canakinumab且hsCRP浓度低于2 mg / L的试验参与者的主要不良心血管事件减少了25%(多变量调整的危险比[HRadj] = 0·75,95%CI 0·66-0·85 ,p <0·0001),而在治疗中hsCRP浓度为2 mg / L或更高的患者中未观察到显着获益(HRadj = 0·90,0·79-1·02,p = 0·11)。对于使用卡那基单抗治疗且治疗中hsCRP浓度低于2 mg / L的患者,心血管疾病死亡率(HRadj = 0·69,95%CI 0·56-0·85,p = 0·0004)和全因死亡率(HRadj = 0·69,0·58-0·81,p <0·0001)均降低了31%,而接受canakinumab治疗且hsCRP浓度达到2 mg / L或以上。在试验中预先设定的次要心血管终点(包括因不稳定心绞痛而需要计划外血运重建的住院治疗)的分析中,或者在基于hsCRP降低的中位数,hsCRP降低的50%或更高的敏感性分析中,也发现了类似的差异作用。 hsCRP降低百分比,剂量特异性分析以及使用因果推论方法进行的分析,以评估达到目标hsCRP浓度的个体的治疗效果。解释单次使用canakinumab后hsCRP降低的幅度可能提供一种简单的临床方法,以鉴定最有可能从持续治疗中获得最大益处的个体。这些数据进一步表明,较低的剂量对使用canakinumab减轻炎症效果更好。资金诺华制药。











































 京公网安备 11010802027423号
京公网安备 11010802027423号