当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
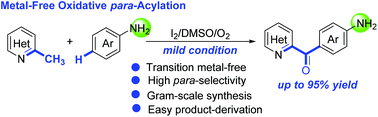
Metal-free oxidative para-acylation of unprotected anilines with N-heteroarylmethanes
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2017-10-25 00:00:00 , DOI: 10.1039/c7ob02490h Min Liu 1, 2, 3, 4, 5 , Xue Chen 1, 2, 3, 4, 5 , Tieqiao Chen 1, 2, 3, 4, 5 , Qing Xu 5, 6, 7, 8 , Shuang-Feng Yin 1, 2, 3, 4, 5
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2017-10-25 00:00:00 , DOI: 10.1039/c7ob02490h Min Liu 1, 2, 3, 4, 5 , Xue Chen 1, 2, 3, 4, 5 , Tieqiao Chen 1, 2, 3, 4, 5 , Qing Xu 5, 6, 7, 8 , Shuang-Feng Yin 1, 2, 3, 4, 5
Affiliation
|
A selective oxidative para-acylation of unprotected anilines with methyl groups in N-heteroarylmethanes was achieved. This transformation proceeds under mild metal-free reaction conditions to produce the corresponding valuable diarylmethanones in good to high yields, featuring high site-selectivity, high functional-group-tolerance, gram-scale synthesis and easy product-derivation. Preliminary mechanistic studies revealed that the present oxidative para-acylation would take place via a Friedel–Crafts-type process of in situ imines and the steric hindrance might be the key issue for the high regio-selectivity.
中文翻译:

未保护的苯胺与N-杂芳基甲烷的无金属氧化对乙酰化
在N-杂芳基甲烷中实现了未保护的苯胺与甲基的选择性氧化对酰化。该转化在温和的无金属反应条件下进行,以高到高收率生产相应的有价值的二芳基亚甲基,具有高的位点选择性,高的官能团耐受性,克级合成和易于衍生的特点。初步的机理研究表明,目前的氧化对酰化反应将通过原位亚胺的Friedel-Crafts型过程进行,并且位阻可能是区域选择性高的关键问题。
更新日期:2017-11-14
中文翻译:

未保护的苯胺与N-杂芳基甲烷的无金属氧化对乙酰化
在N-杂芳基甲烷中实现了未保护的苯胺与甲基的选择性氧化对酰化。该转化在温和的无金属反应条件下进行,以高到高收率生产相应的有价值的二芳基亚甲基,具有高的位点选择性,高的官能团耐受性,克级合成和易于衍生的特点。初步的机理研究表明,目前的氧化对酰化反应将通过原位亚胺的Friedel-Crafts型过程进行,并且位阻可能是区域选择性高的关键问题。









































 京公网安备 11010802027423号
京公网安备 11010802027423号