当前位置:
X-MOL 学术
›
Catal. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Heterocyclic bismuth(III) compounds with transannular N→Bi interactions as catalysts for the oxidation of thiophenol to diphenyldisulfide
Catalysis Science & Technology ( IF 4.4 ) Pub Date : 2017-05-26 00:00:00 , DOI: 10.1039/c7cy00521k Ana M. Toma 1, 2, 3, 4, 5 , Ciprian I. Raţ 1, 2, 3, 4, 5 , Octavian D. Pavel 6, 7, 8, 9, 10 , Christopher Hardacre 11, 12, 13, 14, 15 , Tobias Rüffer 16, 17, 18, 19, 20 , Heinrich Lang 16, 17, 18, 19, 20 , Michael Mehring 16, 17, 19, 20 , Anca Silvestru 1, 2, 3, 4, 5 , Vasile I. Pârvulescu 6, 7, 8, 9, 10
Catalysis Science & Technology ( IF 4.4 ) Pub Date : 2017-05-26 00:00:00 , DOI: 10.1039/c7cy00521k Ana M. Toma 1, 2, 3, 4, 5 , Ciprian I. Raţ 1, 2, 3, 4, 5 , Octavian D. Pavel 6, 7, 8, 9, 10 , Christopher Hardacre 11, 12, 13, 14, 15 , Tobias Rüffer 16, 17, 18, 19, 20 , Heinrich Lang 16, 17, 18, 19, 20 , Michael Mehring 16, 17, 19, 20 , Anca Silvestru 1, 2, 3, 4, 5 , Vasile I. Pârvulescu 6, 7, 8, 9, 10
Affiliation
|
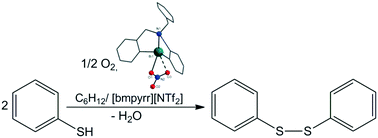
The reactions between the diorganobismuth(III) bromides [RCH2N(CH2C6H4)2]BiBr [R = C6H5 (1), C6H5CH2 (2)] and appropriate silver salts resulted in new diorganobismuth(III) compounds of the general formula [RCH2N(CH2C6H4)2]BiX [R = C6H5, X = ONO2 (3), OSO2CF3 (4), OSO2C6H4 (CHCH2)-4 (5); R = C6H5CH2, X = ONO2 (6)], based on a butterfly-like tetrahydro-dibenzo[c,f][1,5]azabismocine heterocyclic framework. The new species were structurally characterized in solution by 1H, 13C{H} and 19F{H} NMR and in the solid state by IR spectroscopy and single-crystal X-ray diffraction. The nitrogen atom is intramolecularly coordinated to bismuth, thus resulting in hypercoordinated species of type 12-Bi-5 (3 and 6) and 10-Bi-4 (4 and 5). In addition, compound 4 shows bismuth⋯π arene and compounds 3 and 6 bismuth⋯oxygen intermolecular interactions, thus leading to dimers in the solid state. These compounds were investigated as catalysts for the oxidation of thiophenol to diphenyl disulfide by using air as an oxidizing agent, both in cyclohexane and in an ionic liquid (1-butyl-1-methylpyrrolidinium bis(trifluoromethanesulfonyl)imide), at temperatures below 100 °C, affording high reaction rates (TON 34.8, with 100% conversion after 5 h) and a total selectivity to the targeted product.
中文翻译:

具有环N→Bi相互作用的杂环铋(III)化合物可催化将苯硫酚氧化为二苯基二硫化物
溴化二有机铋(III)[RCH 2 N(CH 2 C 6 H 4)2 ] BiBr [R = C 6 H 5(1),C 6 H 5 CH 2(2)]与适当的银盐反应在新diorganobismuth(III通式)化合物[RCH 2 N(CH 2 C ^ 6 ħ 4)2 ] BIX [R = C 6 H ^ 5,X = ONO 2(3),OSO 2 CF 3(4),OSO 2 C 6 H 4(CHCH 2)-4(5);R = C 6 H 5 CH 2,X = ONO 2(6)],基于蝴蝶状的四氢-二苯并[ c,f ] [1,5]氮杂双嘧啶杂环骨架。该新物种在溶液中的结构特征为1 H,13 C {H}和19F {H} NMR,并通过红外光谱和单晶X射线衍射进行固态分析。氮原子在分子内与铋配位,从而产生12-Bi-5(3和6)和10-Bi-4(4和5)类型的超配位物种。此外,化合物4显示铋⋯芳烃,化合物3和6铋氧分子间的相互作用,从而导致固态的二聚体。在低于100°C的温度下,在环己烷和离子液体(1-丁基-1-甲基吡咯烷鎓双(三氟甲磺酰基)酰亚胺)中,通过使用空气作为氧化剂,研究了这些化合物作为将苯酚氧化为二苯基二硫化物的催化剂。 C,提供高反应速率(TON 34.8,5小时后100%转化)和对目标产物的总选择性。
更新日期:2017-11-14
中文翻译:

具有环N→Bi相互作用的杂环铋(III)化合物可催化将苯硫酚氧化为二苯基二硫化物
溴化二有机铋(III)[RCH 2 N(CH 2 C 6 H 4)2 ] BiBr [R = C 6 H 5(1),C 6 H 5 CH 2(2)]与适当的银盐反应在新diorganobismuth(III通式)化合物[RCH 2 N(CH 2 C ^ 6 ħ 4)2 ] BIX [R = C 6 H ^ 5,X = ONO 2(3),OSO 2 CF 3(4),OSO 2 C 6 H 4(CHCH 2)-4(5);R = C 6 H 5 CH 2,X = ONO 2(6)],基于蝴蝶状的四氢-二苯并[ c,f ] [1,5]氮杂双嘧啶杂环骨架。该新物种在溶液中的结构特征为1 H,13 C {H}和19F {H} NMR,并通过红外光谱和单晶X射线衍射进行固态分析。氮原子在分子内与铋配位,从而产生12-Bi-5(3和6)和10-Bi-4(4和5)类型的超配位物种。此外,化合物4显示铋⋯芳烃,化合物3和6铋氧分子间的相互作用,从而导致固态的二聚体。在低于100°C的温度下,在环己烷和离子液体(1-丁基-1-甲基吡咯烷鎓双(三氟甲磺酰基)酰亚胺)中,通过使用空气作为氧化剂,研究了这些化合物作为将苯酚氧化为二苯基二硫化物的催化剂。 C,提供高反应速率(TON 34.8,5小时后100%转化)和对目标产物的总选择性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号