当前位置:
X-MOL 学术
›
Catal. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Highly active Au/δ-MoC and Au/β-Mo2C catalysts for the low-temperature water gas shift reaction: effects of the carbide metal/carbon ratio on the catalyst performance
Catalysis Science & Technology ( IF 5 ) Pub Date : 2017-05-08 00:00:00 , DOI: 10.1039/c7cy00639j Sergio Posada-Pérez 1, 2, 3, 4 , Ramón A. Gutiérrez 5, 6, 7, 8 , Zhijun Zuo 9, 10, 11, 12 , Pedro J. Ramírez 5, 6, 7, 8 , Francesc Viñes 1, 2, 3, 4 , Ping Liu 13, 14, 15, 16 , Francesc Illas 1, 2, 3, 4 , José A. Rodriguez 13, 14, 15, 16
Catalysis Science & Technology ( IF 5 ) Pub Date : 2017-05-08 00:00:00 , DOI: 10.1039/c7cy00639j Sergio Posada-Pérez 1, 2, 3, 4 , Ramón A. Gutiérrez 5, 6, 7, 8 , Zhijun Zuo 9, 10, 11, 12 , Pedro J. Ramírez 5, 6, 7, 8 , Francesc Viñes 1, 2, 3, 4 , Ping Liu 13, 14, 15, 16 , Francesc Illas 1, 2, 3, 4 , José A. Rodriguez 13, 14, 15, 16
Affiliation
|
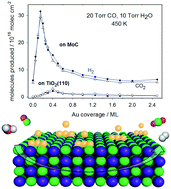
The water gas shift (WGS) reaction catalyzed by orthorhombic β-Mo2C and cubic δ-MoC surfaces with and without Au clusters supported thereon has been studied by means of a combination of sophisticated experiments and state-of-the-art computational modeling. Experiments evidence the importance of the metal/carbon ratio on the performance of these systems, where Au/δ-MoC is presented as a suitable catalyst for WGS at low temperatures owing to its high activity, selectivity (only CO2 and H2 are detected), and stability (oxycarbides are not observed). Periodic density functional theory-based calculations show that the supported Au clusters and the Au/δ-MoC interface do not take part directly in water dissociation but their presence is crucial to switch the reaction mechanism, drastically decreasing the effect of the reverse WGS reaction and favoring the WGS products desorption, thus leading to an increase in CO2 and H2 production. The present results clearly display the importance of the Mo/C ratio and the synergy with the admetal clusters in tuning the activity and selectivity of the carbide substrate.
中文翻译:

高活性的Au /δ-交通部和Au /β-沫2为低温水煤气变换反应C催化剂:对催化剂性能的金属碳化物/碳比率的影响
水煤气变换(WGS)反应由斜方晶系β-Mo系催化2 C和立方δ-邮电部具有和不具有金团簇支撑在其上进行了研究的复杂的实验和国家的最先进的计算建模的组合来表面。实验证明了金属/碳比对这些系统的性能的重要性,其中Au /δ-MoC由于其高活性,高选择性(仅CO 2和H 2)而在低温下作为WGS的合适催化剂。检测)和稳定性(未观察到碳氧化物)。基于周期密度泛函理论的计算表明,所支持的Au团簇和Au /δ-MoC界面不直接参与水离解,但它们的存在对于切换反应机理至关重要,从而大大降低了WGS逆反应和有利于WGS产物的解吸,从而导致CO 2和H 2产生的增加。目前的结果清楚地表明了Mo / C比和与亚金属簇的协同作用在调节碳化物基体的活性和选择性方面的重要性。
更新日期:2017-11-14
中文翻译:

高活性的Au /δ-交通部和Au /β-沫2为低温水煤气变换反应C催化剂:对催化剂性能的金属碳化物/碳比率的影响
水煤气变换(WGS)反应由斜方晶系β-Mo系催化2 C和立方δ-邮电部具有和不具有金团簇支撑在其上进行了研究的复杂的实验和国家的最先进的计算建模的组合来表面。实验证明了金属/碳比对这些系统的性能的重要性,其中Au /δ-MoC由于其高活性,高选择性(仅CO 2和H 2)而在低温下作为WGS的合适催化剂。检测)和稳定性(未观察到碳氧化物)。基于周期密度泛函理论的计算表明,所支持的Au团簇和Au /δ-MoC界面不直接参与水离解,但它们的存在对于切换反应机理至关重要,从而大大降低了WGS逆反应和有利于WGS产物的解吸,从而导致CO 2和H 2产生的增加。目前的结果清楚地表明了Mo / C比和与亚金属簇的协同作用在调节碳化物基体的活性和选择性方面的重要性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号