当前位置:
X-MOL 学术
›
J. Mater. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Controlling Cu2ZnSnS4 (CZTS) phase in microwave solvothermal synthesis
Journal of Materials Chemistry A ( IF 10.7 ) Pub Date : 2017-10-18 00:00:00 , DOI: 10.1039/c7ta06086f Alexandre H. Pinto 1, 2, 3, 4 , Seung Wook Shin 2, 3, 4, 5 , Elianna Isaac 1, 2, 3, 4 , Theodore R. Knutson 1, 2, 3, 4 , Eray S. Aydil 2, 3, 4, 5 , R. Lee Penn 1, 2, 3, 4
Journal of Materials Chemistry A ( IF 10.7 ) Pub Date : 2017-10-18 00:00:00 , DOI: 10.1039/c7ta06086f Alexandre H. Pinto 1, 2, 3, 4 , Seung Wook Shin 2, 3, 4, 5 , Elianna Isaac 1, 2, 3, 4 , Theodore R. Knutson 1, 2, 3, 4 , Eray S. Aydil 2, 3, 4, 5 , R. Lee Penn 1, 2, 3, 4
Affiliation
|
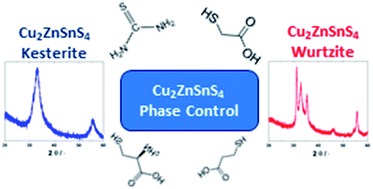
The semiconductor Cu2ZnSnS4 (CZTS) is a promising sustainable photovoltaic material and colloidal dispersions of wurtzite or kesterite CZTS nanocrystals are often used to make thin polycrystalline films for solar cells. This requires control of the nanocrystal phases via the synthesis. We studied the microwave-assisted solvothermal synthesis of CZTS nanocrystals from metal salts and thiourea in ethylene glycol in the presence of various excess sulfur sources. Relative fractions of the kesterite and wurtzite phases depend on the excess sulfur source, the oxidation state of Sn, and the sulfur-to-total-metal -cation (S : M) ratio used in the synthesis. When the excess sulfur source contains an amino group, a Zn–Sn intermediate forms and allows the CZTS phase to be varied between kesterite and wurtzite via the Sn initial oxidation state and S : M ratio. When the excess sulfur source contains an amino group, synthesis using Sn(II) salts and low S : M (1.9) favors the formation of the wurtzite phase, whereas synthesis using high S : M ratio (>4) favors the formation of the kesterite phase. Only the kesterite phase is obtained when Sn(IV) reagent is used, regardless of S : M ratio. When the excess sulfur source does not have an amino group, only the wurtzite phase is obtained under the conditions studied, regardless of the oxidation state of the Sn precursor or the S : M ratio; in these cases, the Zn–Sn intermediate does not form and the precursor to wurtzite appears to be copper sulfide.
中文翻译:

微波溶剂热合成中Cu 2 ZnSnS 4(CZTS)相的控制
半导体Cu 2 ZnSnS 4(CZTS)是一种有前途的可持续光伏材料,纤锌矿或钾长石CZTS纳米晶体的胶体分散体通常用于制造太阳能电池薄膜。这需要通过以下方式控制纳米晶相综合。我们研究了在各种过量硫源存在下,在乙二醇中微波辅助溶剂热法从乙二醇中的金属盐和硫脲合成CZTS纳米晶体的方法。钾长石和纤锌矿相的相对分数取决于过量的硫源,Sn的氧化态以及合成中使用的硫与金属阳离子的总比(S:M)。当过量的硫源包含氨基时,会形成Zn-Sn中间体,并通过Sn的初始氧化态和S:M比率使CZTS相在钾长石和纤锌矿之间变化。当过量的硫源包含氨基时,使用Sn(II)盐和低S:M(1.9)有利于纤锌矿相的形成,而使用高S:M比(> 4)进行合成则有利于钾长石相的形成。当使用Sn(IV)试剂时,无论S:M的比例如何,仅获得钾长石相。当过量的硫源不具有氨基时,在所研究的条件下仅获得纤锌矿相,而与Sn前体的氧化态或S∶M比无关。在这些情况下,不会形成Zn-Sn中间体,纤锌矿的前体似乎是硫化铜。
更新日期:2017-11-14
中文翻译:

微波溶剂热合成中Cu 2 ZnSnS 4(CZTS)相的控制
半导体Cu 2 ZnSnS 4(CZTS)是一种有前途的可持续光伏材料,纤锌矿或钾长石CZTS纳米晶体的胶体分散体通常用于制造太阳能电池薄膜。这需要通过以下方式控制纳米晶相综合。我们研究了在各种过量硫源存在下,在乙二醇中微波辅助溶剂热法从乙二醇中的金属盐和硫脲合成CZTS纳米晶体的方法。钾长石和纤锌矿相的相对分数取决于过量的硫源,Sn的氧化态以及合成中使用的硫与金属阳离子的总比(S:M)。当过量的硫源包含氨基时,会形成Zn-Sn中间体,并通过Sn的初始氧化态和S:M比率使CZTS相在钾长石和纤锌矿之间变化。当过量的硫源包含氨基时,使用Sn(II)盐和低S:M(1.9)有利于纤锌矿相的形成,而使用高S:M比(> 4)进行合成则有利于钾长石相的形成。当使用Sn(IV)试剂时,无论S:M的比例如何,仅获得钾长石相。当过量的硫源不具有氨基时,在所研究的条件下仅获得纤锌矿相,而与Sn前体的氧化态或S∶M比无关。在这些情况下,不会形成Zn-Sn中间体,纤锌矿的前体似乎是硫化铜。











































 京公网安备 11010802027423号
京公网安备 11010802027423号