当前位置:
X-MOL 学术
›
ChemCatChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Zeolite‐Catalyzed Formaldehyde–Propylene Prins Condensation
ChemCatChem ( IF 4.5 ) Pub Date : 2017-11-13 , DOI: 10.1002/cctc.201701315 Efterpi S. Vasiliadou 1 , Nicholas S. Gould 1, 2 , Raul F. Lobo 1, 2
ChemCatChem ( IF 4.5 ) Pub Date : 2017-11-13 , DOI: 10.1002/cctc.201701315 Efterpi S. Vasiliadou 1 , Nicholas S. Gould 1, 2 , Raul F. Lobo 1, 2
Affiliation
|
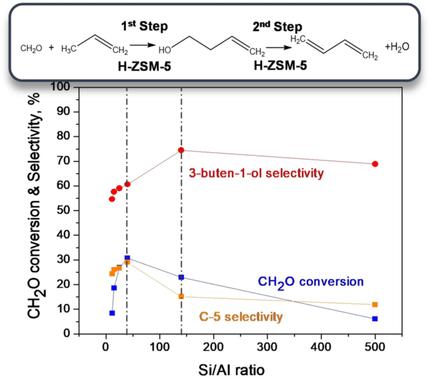
Prins condensation of formaldehyde with propylene to form 3‐buten‐1‐ol is investigated using microporous solid acid catalysts. Zn/H‐beta shows high conversion but leads to a broad product distribution composed primarily of pyrans. Mechanistic studies revealed that 3‐buten‐1‐ol reacts via Prins cyclization or dehydrate to 1,3‐butadiene that further reacts with formaldehyde via a hetero‐Diels–Alder reaction. These secondary reactions are suppressed over ZSM‐5 catalysts: 3‐buten‐1‐ol is the predominant product over H‐ZSM‐5 zeolite under all conditions investigated. 3‐Buten‐1‐ol selectivity of up to 75 % is achieved. In a second step 3‐buten‐1‐ol dehydrates at temperatures as low as 423 K, forming 1,3‐butadiene. Although Brønsted acid sites are the primary catalytic sites, ion exchange of ZnII increases the overall rate and 3‐buten‐1‐ol selectivity. H‐ZSM‐5 showed significant differences in reactivity and selectivity as a function of the Si/Al ratio; optimal catalytic properties were observed within Si/Al=40–140.
中文翻译:

沸石催化的甲醛-丙烯缩聚物的缩合
使用微孔固体酸催化剂研究了甲醛与丙烯的缩聚反应,形成3-丁烯-1-醇。Zn /H-β显示出高转化率,但导致主要由吡喃组成的广泛产品分布。机理研究表明,3-丁烯-1-醇通过Prins环化反应或脱水成1,3-丁二烯,然后通过杂Diels-Alder反应与甲醛进一步反应。这些次级反应在ZSM-5催化剂上得到抑制:在所有研究的条件下,3-丁烯-1-醇是H-ZSM-5沸石的主要产物。达到了最高75%的3-丁烯-1-醇选择性。第二步,3-丁烯-1-醇在低至423 K的温度下脱水,形成1,3-丁二烯。尽管布朗斯台德酸位点是主要的催化位点,但Zn II的离子交换提高了总转化率和3-丁烯-1-醇的选择性。H‐ZSM-5的反应性和选择性显着不同,这是Si / Al比的函数。在Si / Al = 40-140范围内观察到最佳的催化性能。
更新日期:2017-11-13
中文翻译:

沸石催化的甲醛-丙烯缩聚物的缩合
使用微孔固体酸催化剂研究了甲醛与丙烯的缩聚反应,形成3-丁烯-1-醇。Zn /H-β显示出高转化率,但导致主要由吡喃组成的广泛产品分布。机理研究表明,3-丁烯-1-醇通过Prins环化反应或脱水成1,3-丁二烯,然后通过杂Diels-Alder反应与甲醛进一步反应。这些次级反应在ZSM-5催化剂上得到抑制:在所有研究的条件下,3-丁烯-1-醇是H-ZSM-5沸石的主要产物。达到了最高75%的3-丁烯-1-醇选择性。第二步,3-丁烯-1-醇在低至423 K的温度下脱水,形成1,3-丁二烯。尽管布朗斯台德酸位点是主要的催化位点,但Zn II的离子交换提高了总转化率和3-丁烯-1-醇的选择性。H‐ZSM-5的反应性和选择性显着不同,这是Si / Al比的函数。在Si / Al = 40-140范围内观察到最佳的催化性能。



























 京公网安备 11010802027423号
京公网安备 11010802027423号