当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cu-Catalyzed asymmetric Friedel–Crafts propargylic alkylation of phenol derivatives
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2017-11-06 00:00:00 , DOI: 10.1039/c7ob02133j Long Shao 1, 2, 3, 4, 5 , Xiang-Ping Hu 1, 2, 3, 4
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2017-11-06 00:00:00 , DOI: 10.1039/c7ob02133j Long Shao 1, 2, 3, 4, 5 , Xiang-Ping Hu 1, 2, 3, 4
Affiliation
|
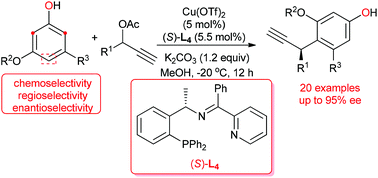
A copper-catalyzed asymmetric Friedel–Crafts propargylic alkylation of electron-rich phenol derivatives with a variety of propargylic esters has been described. With Cu(OTf)2 decorated with a chiral tridentate ketimine P,N,N-ligand as the catalyst, asymmetric Friedel–Crafts propargylic alkylation of 3,5-dialkoxyphenol derivatives proceeded smoothly in high yields and with good to excellent enantioselectivities. The present study suggested that the presence of an electron-rich substituent on the meta-position of phenol is essential for the promotion of Friedel–Crafts propargylic alkylation, and the substrate bearing two electron-rich groups on both the 3,5-positions of phenol tends to give a satisfactory performance.
中文翻译:

铜催化苯酚衍生物的不对称Friedel-Crafts炔丙基烷基化
已经描述了铜催化的富电子苯酚衍生物与各种炔丙基酯的不对称Friedel-Crafts炔丙基烷基化反应。用手性三齿酮亚胺酮P,N,N-配体作为催化剂装饰的Cu(OTf)2,不对称的Friedel-Crafts 3,5-二烷氧基苯酚衍生物的炔丙基烷基化反应顺利进行,收率很高,对映选择性很好。本研究表明,在苯酚的间位上存在一个富电子取代基对于促进Friedel-Crafts炔丙基烷基化是必不可少的,并且底物在两个3,5-位上均带有两个富电子基团苯酚倾向于提供令人满意的性能。
更新日期:2017-11-13
中文翻译:

铜催化苯酚衍生物的不对称Friedel-Crafts炔丙基烷基化
已经描述了铜催化的富电子苯酚衍生物与各种炔丙基酯的不对称Friedel-Crafts炔丙基烷基化反应。用手性三齿酮亚胺酮P,N,N-配体作为催化剂装饰的Cu(OTf)2,不对称的Friedel-Crafts 3,5-二烷氧基苯酚衍生物的炔丙基烷基化反应顺利进行,收率很高,对映选择性很好。本研究表明,在苯酚的间位上存在一个富电子取代基对于促进Friedel-Crafts炔丙基烷基化是必不可少的,并且底物在两个3,5-位上均带有两个富电子基团苯酚倾向于提供令人满意的性能。











































 京公网安备 11010802027423号
京公网安备 11010802027423号