当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
One-step methodology for the direct covalent capture of GPCRs from complex matrices onto solid surfaces based on the bioorthogonal reaction between haloalkane dehalogenase and chloroalkanes
Chemical Science ( IF 7.6 ) Pub Date : 2017-10-19 00:00:00 , DOI: 10.1039/c7sc03887a Kaizhu Zeng 1, 2, 3, 4, 5 , Qian Li 1, 2, 3, 4, 5 , Jing Wang 1, 2, 3, 4, 5 , Guowei Yin 6, 7, 8 , Yajun Zhang 1, 2, 3, 4, 5 , Chaoni Xiao 1, 2, 3, 4, 5 , Taiping Fan 1, 2, 3, 4, 5 , Xinfeng Zhao 1, 2, 3, 4, 5 , Xiaohui Zheng 1, 2, 3, 4, 5
Chemical Science ( IF 7.6 ) Pub Date : 2017-10-19 00:00:00 , DOI: 10.1039/c7sc03887a Kaizhu Zeng 1, 2, 3, 4, 5 , Qian Li 1, 2, 3, 4, 5 , Jing Wang 1, 2, 3, 4, 5 , Guowei Yin 6, 7, 8 , Yajun Zhang 1, 2, 3, 4, 5 , Chaoni Xiao 1, 2, 3, 4, 5 , Taiping Fan 1, 2, 3, 4, 5 , Xinfeng Zhao 1, 2, 3, 4, 5 , Xiaohui Zheng 1, 2, 3, 4, 5
Affiliation
|
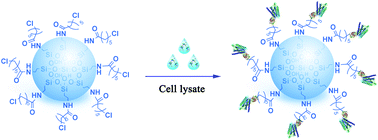
Protein immobilization techniques play an important role in the development of assays for disease diagnosis and drug discovery. However, many of these approaches are not applicable to transmembrane proteins. G protein-coupled receptors (GPCRs) are the largest protein superfamily encoded by the human genome and are targeted by a quarter of all prescription drugs. GPCRs are highly dynamic and sensitive to changes in the ambient environment, and current immobilization methodologies are not suitable for GPCRs. We used haloalkane dehalogenase (Halo) as an immobilization tag fused to the β2-adrenoceptor (β2-AR), angiotensin II type 1 (AT1) and angiotensin II type 2 (AT2) receptors. The engineered Halo-tag covalently binds to a specific substrate chloroalkane through Asp 106 in the catalytic pocket. The Halo-tagged GPCRs were expressed in Escherichia coli at a suitable yield. Accordingly, we loaded cell lysate containing Halo-tagged GPCRs onto a macroporous silica gel coated with chloroalkane. Morphological characterization indicated a homogeneous monolayer of immobilized Halo-tagged GPCRs on the silica gel surface. The immobilized receptors proved to be surrounded by specific bound phospholipids including PG C18:1/C18:1. We observed a radio-ligand binding ability and ligand-induced conformational changes in the immobilized GPCRs, suggesting the preservation of bioactivity. This method is a one-step approach for the specific immobilization of GPCRs from cell lysates and validates that immobilized receptors retain canonical ligand binding capacity. Our immobilization strategy circumvents labor-intensive purification procedures and minimizes loss of activity. The immobilized receptors can be applied to high-throughput drug and interaction partner screening for GPCRs.
中文翻译:

基于卤代烷脱卤酶和氯代烷之间的生物正交反应,从复杂基质直接共价捕获GPCR到固体表面的一步法
蛋白质固定技术在疾病诊断和药物发现检测方法的开发中起着重要作用。然而,这些方法中的许多不适用于跨膜蛋白。G蛋白偶联受体(GPCR)是人类基因组编码的最大蛋白超家族,被所有处方药的四分之一靶向。GPCR是高度动态的,并且对周围环境的变化敏感,并且当前的固定化方法不适用于GPCR。我们使用卤代烷脱卤素(卤代)作为融合到β的固定标签2肾上腺素能受体(β 2 -AR),血管紧张素II类型1(AT 1)和血管紧张素II型2(AT 2)受体。经过改造的Halo-tag通过催化口袋中的Asp 106共价结合至特定的底物氯代烷烃。带有Halo标签的GPCR在大肠杆菌中表达以合适的产量。因此,我们将含有Halo标签的GPCR的细胞裂解液加载到涂有氯烷的大孔硅胶上。形态学表征表明硅胶表面上固定有Halo标记GPCR的均质单层。固定的受体被证明被特异性结合的磷脂包围,包括PG C18:1 / C18:1。我们观察到固定的GPCRs中的放射性-配体结合能力和配体诱导的构象变化,表明生物活性的保存。此方法是从细胞裂解物中特异性固定GPCR的一步方法,并验证了固定的受体保留了规范的配体结合能力。我们的固定化策略规避了劳动强度大的纯化程序,并最大程度地减少了活性损失。
更新日期:2017-11-13
中文翻译:

基于卤代烷脱卤酶和氯代烷之间的生物正交反应,从复杂基质直接共价捕获GPCR到固体表面的一步法
蛋白质固定技术在疾病诊断和药物发现检测方法的开发中起着重要作用。然而,这些方法中的许多不适用于跨膜蛋白。G蛋白偶联受体(GPCR)是人类基因组编码的最大蛋白超家族,被所有处方药的四分之一靶向。GPCR是高度动态的,并且对周围环境的变化敏感,并且当前的固定化方法不适用于GPCR。我们使用卤代烷脱卤素(卤代)作为融合到β的固定标签2肾上腺素能受体(β 2 -AR),血管紧张素II类型1(AT 1)和血管紧张素II型2(AT 2)受体。经过改造的Halo-tag通过催化口袋中的Asp 106共价结合至特定的底物氯代烷烃。带有Halo标签的GPCR在大肠杆菌中表达以合适的产量。因此,我们将含有Halo标签的GPCR的细胞裂解液加载到涂有氯烷的大孔硅胶上。形态学表征表明硅胶表面上固定有Halo标记GPCR的均质单层。固定的受体被证明被特异性结合的磷脂包围,包括PG C18:1 / C18:1。我们观察到固定的GPCRs中的放射性-配体结合能力和配体诱导的构象变化,表明生物活性的保存。此方法是从细胞裂解物中特异性固定GPCR的一步方法,并验证了固定的受体保留了规范的配体结合能力。我们的固定化策略规避了劳动强度大的纯化程序,并最大程度地减少了活性损失。







































 京公网安备 11010802027423号
京公网安备 11010802027423号